Good morning, everyone. Thank you, Rob, for your leadership of the American Bankers Association. I greatly appreciate the invitation to be with all of you at an important moment.
As everyone in this room knows, the American economy relies on a healthy banking system that can provide for the credit needs of families and businesses. American households depend on banks to finance their homes, invest in an education, and otherwise improve their standards of living. Businesses borrow from these institutions to start new companies and expand existing ones.
I. RECENT DEVELOPMENTS IN THE BANKING SYSTEM
Almost two weeks ago, we learned of problems at two banks that could have had significant impacts on the broader banking system and the economy. The situation demanded a swift response. In the days that followed, the federal government delivered just that: decisive and forceful actions to strengthen public confidence in the U.S. banking system and protect the American economy.
Let me be clear: the government’s recent actions have demonstrated our resolute commitment to take the necessary steps to ensure that depositors’ savings and the banking system remain safe.
Our approach had two main pillars.
First, we worked with the Federal Reserve and FDIC to protect all depositors in the resolutions of Silicon Valley Bank and Signature Bank. The steps we took were not focused on aiding specific banks or classes of banks. Our intervention was necessary to protect the broader U.S. banking system. And similar actions could be warranted if smaller institutions suffer deposit runs that pose the risk of contagion. I believe that our actions reduced the risk of further bank failures that would have imposed losses on the Deposit Insurance Fund, which is paid for through fees on insured banks.
Second, we announced a new facility to provide additional liquidity to the banking system. The Fed’s new lending facility – the Bank Term Funding Program – is designed to help banks meet the needs of all of their depositors.
The situation is stabilizing. And the U.S. banking system remains sound. The Fed facility and discount window lending are working as intended to provide liquidity to the banking system. Aggregate deposit outflows from regional banks have stabilized.
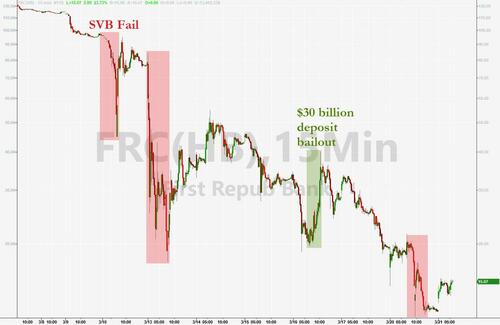
As you know, 11 banks – including the very largest and some regional banks – announced $30 billion in deposits into First Republic Bank last week. This support represents a vote of confidence in our banking system.
We are continuing to monitor conditions closely. My team and I have been in close communication with many of you, in addition to federal and state regulators, other market participants, and international counterparts.
While we don’t yet have all the details about the collapse of the two banks, we do know that the recent developments are very different than those of the Global Financial Crisis. Back then, many financial institutions came under stress due to their holdings of subprime assets. We do not see that situation in the banking system today. Our financial system is also significantly stronger than it was 15 years ago. This is in large part due to post-crisis reforms that provided stronger capital standards, among other important improvements.
In the coming weeks, it will be vital for us to get a full accounting of exactly what happened in these bank failures. Regulators have already announced a review into Silicon Valley Bank. We are currently focused on the situation at hand. But we will need to reexamine our current regulatory and supervisory regimes and consider whether they are appropriate for the risks that banks face today. We all share an interest in ensuring that the United States remains the strongest and safest financial system in the world.
II. IMPORTANCE OF A BROAD AND DIVERSE BANKING SYSTEM
Given recent developments, I think it is important to reaffirm a broader point: our dynamic and diverse banking system is critical to the American economy. Large banks play an important role in our economy, but so do small- and mid-sized banks. These banks are heavily engaged in traditional banking services that provide vital credit and financial support to families and small businesses. They also increase competition in the banking sector, and often have specialized knowledge and expertise in the communities they invest in.
Indeed, many of these banks have played an important role in supporting our economic recovery. In the depths of the pandemic, Treasury was tasked with getting money quickly and responsibly to those who needed it. So, we worked closely with many of you to send economic impact payments to millions of families. You’ve also worked with us to deliver advance payments from the enhanced Child Tax Credit – which helped cut child poverty nearly in half in 2021. And we’ve collaborated to rapidly deploy assistance to hundreds of thousands of homeowners facing foreclosure.
I also know that many of you are working with state governments to inject new financing into small businesses as part of our State Small Business Credit Initiative. In the prior iteration of this program, lenders with less than $10 billion in assets accounted for 95 percent of all program-supported loans. Our Administration has also been focused on working with mission-oriented banks to put us on a path toward inclusive economic growth. For example, Treasury’s Emergency Capital Investment Program has invested almost $8.4 billion in depository institutions that are CDFIs and MDIs.
Treasury is committed to ensuring the ongoing health and competitiveness of our vibrant community and regional banking institutions.
III. CONCLUSION
To end, let me return to where I started. A safe and sound banking system is integral to the health of the American economy. We are squarely focused on doing our job. And you should rest assured that we will remain vigilant.
I look forward to continuing to work together to strengthen our banking system and our nation’s economy.
Thank you.
* * *
As we detailed earlier, the sound and fury of demands for universal deposit insurance are growing with Bill Ackman and Elon Musk the latest to join the calls for this ultimate step and as we detailed last night, Bloomberg reports Washington is studying just how to guarantee all $18 trillion in US deposits (with just $125 billion in the FDIC's Deposit Insurance Fund).
"US officials are studying ways they might temporarily expand Federal Deposit Insurance Corp. coverage to all deposits, a move sought by a coalition of banks arguing that it’s needed to head off a potential financial crisis."
But since US Treasury Secretary Janet Yellen's embarrassing and confusing comments about who gets saved and who doesn't (and who decides) during testimony before the Senate Finance Committee, it appears Washington thinks trotting out the little old lady once more to reassure nervous depositors is the right path back to financial stability.

As Reuters reports, in excerpts of prepared remarks to an American Bankers Association conference, Yellen said government steps taken in recent days to protect uninsured deposits in two failed banks and create new Federal Reserve liquidity facilities have shown a "resolute commitment to take the necessary steps to ensure that depositors’ savings and the banking system remain safe."
But she immediately switches to try to quell any 'picking winners' sentiment by assuring her audience that:
“The steps we took were not focused on aiding specific banks or classes of banks. Our intervention was necessary to protect the broader U.S. banking system,” Yellen said.
And crucially, she claims they will rescue ALL depositors again...
“And similar actions could be warranted if smaller institutions suffer deposit runs that pose the risk of contagion.”
Of course, there is the obligatory - 'do not worry, there is nothing to see here' comment (or "it's contained")...
“The situation is stabilizing. And the U.S. banking system remains sound,” Yellen said.
“The Fed facility and discount window lending are working as intended to provide liquidity to the banking system. Aggregate deposit outflows from regional banks have stabilized.”
Tell that to First Republic Bank shareholders...
Specifically, the Treasury chief didn’t directly address the issue of temporarily expanding federal deposit insurance to cover all deposits in the excerpts released by the Treasury Department.
“Treasury is committed to ensuring the ongoing health and competitiveness of our vibrant community and regional banking institutions,” she said.
Of course, the only path to this is actual legislation, which means Congress (i.e. no executive order)...
“Any universal guarantee on all bank deposits, whether implicit or explicit, enshrines a dangerous precedent that simply encourages future irresponsible behavior to be paid for by those not involved who followed the rules,” the House Freedom Caucus said in a statement, and we are confident most of the progressive wing will not be too excited about bailing out billionaires and corporations with orders of magnitude more in the bank than the FDIC limit.
...and that means, don't hold your breath for any blanket unlimited size deposit insurance.
https://www.zerohedge.com/markets/yellen-reassure-world-treasury-committed-bailing-out-regional-bank-depositors