Search This Blog
Monday, September 10, 2018
Sanofi rating change at BofA/Merrill
Sanofi upgraded to Buy from Neutral at BofA/Merrill. BofA/Merrill analyst Graham Parry upgraded Sanofi to Buy from Neutral saying the company is approaching an inflection in growth from new products, mainly Dupixent, atopic, dermatitis, while diabetes becomes less of a headwind. Parry believes sales and earnings growth will accelerate to 7% from 4% in through 20-2023E and views valuation as undervaluing growth.
https://thefly.com/landingPageNews.php?id=2787903
Eli Lilly initiates head-to-head clinical trial for psoriasis med
Eli Lilly and Company announced the initiation of the IXORA-R head-to-head clinical trial, designed to evaluate superiority between Taltz and Tremfya in adult patients with moderate-to-severe plaque psoriasis. The IXORA-R study will be the first H2H trial between an IL-17 and IL-23 using the Psoriasis Area Severity Index 100 score as the primary endpoint. The IXORA-R study, which is set to be completed by the end of 2019, aims to enroll 960 patients and is a 24-week multicenter, randomized, blinded, parallel-group study comparing the efficacy and safety of Taltz to Tremfya in patients with moderate-to-severe plaque psoriasis. The primary endpoint of the study is the proportion of patients who achieve 100% improvement from their baseline as measured by PASI 100 at week 12. Secondary endpoints include: the proportion of patients achieving PASI 75 as early as week 2 and PASI 100 at weeks 4, 8 and 24; and the proportion of patients achieving a Static Physician Global Assessment score of 0 at week 12.
https://thefly.com/landingPageNews.php?id=2787937
Aridis Pharmaceuticals initiated at Northland
Aridis Pharmaceuticals initiated with an Outperform at Northland. Northland analyst Carl Byrns initiated Aridis Pharmaceuticals with an Outperform and $40 price target.
https://thefly.com/landingPageNews.php?id=2787949
Zogenix Phase 2 Study Show Durable Reduction in Seizure Frequency
67% of Patients Achieved At Least a 50% Reduction in Convulsive Seizures
Global Phase 3 Randomized, Controlled Study in LGS Currently Enrolling Patients
Zogenix, Inc. (NASDAQ:ZGNX), a pharmaceutical company developing therapies for the treatment of rare central nervous system (CNS) disorders, today announced that detailed results of the Phase 2, open-label study evaluating its investigational drug, ZX008 (low-dose fenfluramine hydrochloride), for the treatment of refractory patients with Lennox-Gastaut Syndrome (LGS), were published in the September 2018 issue of Epilepsia. Consistent with the previously-reported data from this study, the results demonstrated that ZX008 provided sustained, clinically meaningful seizure reduction in the majority of patients and was generally well-tolerated.
“This study was designed to confirm the potential effectiveness of ZX008 for patients with LGS,” said Lieven Lagae, M.D., Ph.D., lead study author and professor at the University of Leuven, Belgium, head of the Pediatric Neurology Department and director of the Childhood Epilepsy Program at the University of Leuven Hospitals. “The clinically meaningful reduction in convulsive seizures in this highly refractory patient cohort emphasizes the importance of the ongoing Phase 3 study of ZX008 as a treatment for LGS patients, who continue to struggle with seizure control despite current therapies.”
The single-center, Phase 2, open-label dose-finding trial was a 20-week core study and a long-term extension option for those patients who were responders in the core study. Results presented in the paper included up to 15 months of treatment for patients in the long-term extension. Patients initiated treatment of 0.2 mg/kg/day ZX008 twice-daily. Patients who were responders (i.e., achieved > 50% reduction in convulsive seizure frequency after 4 weeks) remained at their effective dose, while non-responders were considered for a dose increase up to 0.8 mg/kg/day at 0.2 mg/kg increments per 4 weeks.
TESARO Moves to Second Stage of Trial of Lung Cancer Combo
- All evaluable patients experienced tumor shrinkage
- Protocol defined response criteria achieved and trial expansion ongoing
Tesaro, Inc. (NASDAQ: TSRO), an oncology-focused biopharmaceutical company, today announced it has initiated the second stage of the JASPER study that is designed to assess clinical benefit of ZEJULA® in combination with an anti-PD-1 antibody in first-line non-small cell lung cancer (NSCLC) patients. The decision to advance the trial was based on achieving the protocol defined response criteria in the initial cohort of 16 treated patients with high PD-L1 expression, of which 14 were evaluable for a response. Nine of the 14 patients had objective responses by RECIST criteria at the time of the analysis1; with all 14 patients experiencing tumor shrinkage.
“These JASPER data provide preliminary evidence that the combination of ZEJULA and an anti-PD-1 antibody could be active as a first-line treatment for patients with non-small cell lung cancer and high levels of PD-L1 expression,” said Mary Lynne Hedley, Ph.D., President and COO of TESARO. “In the second stage of the trial, 36 additional patients will be enrolled and treated with ZEJULA in combination with TSR-042, our anti-PD-1 antibody. TSR-042 is the foundation of our lung cancer strategy, and is also being studied as a monotherapy in our GARNET trial in anti-PD-(L)1 naïve patients who have progressed on chemotherapy, and in combination with TSR-022, our anti-TIM-3 antibody, in AMBER, a study in late-line NSCLC patients that have progressed after anti-PD-(L)1 therapy. We look forward to sharing lung cancer data from both GARNET and AMBER at the Society for the Immunotherapy of Cancer (SITC) Annual Meeting in November.”
Chi-Med Gets OK on Capsules for Colorectal Cancer in China
– Fruquintinib capsules provide a new oral treatment option for patients with metastatic colorectal cancer and will be marketed as Elunate® –
– Elunate® data published in JAMA demonstrated increased overall survival versus placebo –
– First ever approval of an innovative medicine by Chi-Med –
– The first China-discovered and developed treatment for CRC approved in China –
Hutchison China MediTech Limited (“Chi-Med”) (AIM/Nasdaq: HCM) today announces that fruquintinib capsules have been granted approval for drug registration by the National Medical Products Administration of China (“NMPA”, formerly the China Food and Drug Administration) for the treatment of metastatic colorectal cancer (“CRC”) patients, who have failed at least two prior systemic antineoplastic therapies including fluoropyrimidine, oxaliplatin and irinotecan, with or without prior use of anti-vascular endothelial growth factor (“VEGF”) or anti-epidermal growth factor receptor (“EGFR”) therapies. Fruquintinib is a highly selective and potent small molecule oral inhibitor of vascular endothelial growth factor receptors (“VEGFR”) 1, 2 and 3 designed to be a global best-in-class VEGFR inhibitor for many types of solid tumors. Fruquintinib capsules are to be marketed in China under the brand name Elunate®. The approval is based on results from the Phase III FRESCO trial, which were presented at the American Society of Clinical Oncology 2017 Meeting and published in the JAMA (Journal of the American Medical Association) in 2018.
“Today’s approval is a major achievement for Chi-Med,” said Simon To, Chairman of Chi-Med. “Elunate® is the first home-grown, China-discovered and developed drug we are aware of in an oncology indication to be unconditionally approved through a randomized clinical trial in China,” he added, “This is the result of over a dozen years of steadfast commitment by Chi-Med in research and development in China’s emerging biotech ecosystem.”
“We are particularly grateful to the patients, their families, investigators, nurses, caregivers and study team members who participated in the clinical development of Elunate® and now look forward to making this world-class new therapy available as quickly as possible to patients with CRC in China.”
Masimo Gets FDA OK of Acoustic Monitor for Infant and Neonatal Patients
Masimo Corporation (NASDAQ: MASI) announced today FDA clearance of RAS-45, an acoustic respiration sensor for rainbow Acoustic Monitoring® (RAM®), for infant and neonatal patients. RAM could previously be used to monitor adult and pediatric patients greater than 10 kg using RAS-125c and RAS-45 sensors. With clearance of the RAS-45 sensor for infant and neonatal patients, acoustic respiration rate measurement is now, for the first time, possible for patients of all sizes, including neonates, in the United States.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20180910005179/en/
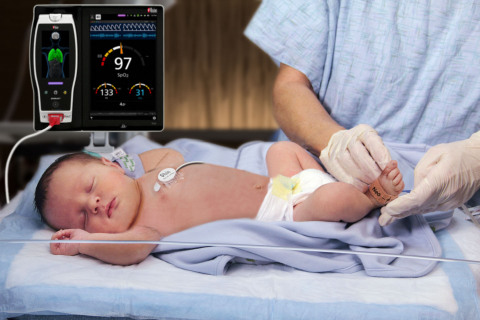 Masimo Root® with Radical-7®, RRa®, and the RAS-45 Infant/Neonatal Sensor (Photo: Business Wire)
Masimo Root® with Radical-7®, RRa®, and the RAS-45 Infant/Neonatal Sensor (Photo: Business Wire)
RAM noninvasively and continuously measures respiration rate using an innovative adhesive sensor with an integrated acoustic transducer, the RAS-45 and RAS-125c, applied to the patient’s neck area or, for infant and neonatal patients under 10 kg, the chest. Using acoustic signal processing that leverages Masimo Signal Extraction Technology® (SET®), the respiratory signal is separated and processed to display continuous respiration rate (RRa®) and an acoustic respiration waveform, a visualization of the vibrations caused by the patient’s airflow. The acoustic sensor also allows clinicians to listen to the sound of a patient’s breathing, whether at the bedside, through a point-of-care device like the Radical-7® Pulse CO-Oximeter®, or remotely, from a Patient SafetyNet™ view station.
The RAS-45 sensor for infant and neonatal patients offers multiple benefits of particular importance for successfully monitoring these youngest and most fragile patients. With the clearance for newborns and neonates, RRa’s accuracy range has been expanded up to 120 breaths per minute, while still providing accuracy of +/- 1 breath per minute, facilitating accurate measurement of the higher respiratory rates common in this population. The sensor itself is significantly smaller than the RAS-125c sensor, and in fact with a diameter of approximately 2.2 cm without adhesive is only slightly larger than a nickel. Similarly, it weighs so little, 13 grams, that its presence may be barely noticeable, and features an adhesive that is transparent, light, and flexible. The size, weight, and adhesive advantages make it particularly suitable for the smaller stature and delicate skin of infants and neonates.
RRa has been shown not only to be accurate1,2 and reliable1, but also easy-to-use1, easy-to-tolerate1,3, and to enhance patient compliance with respiration monitoring. In a study comparing pediatric patient tolerance of sidestream capnography with a nasal cannula to respiration rate monitoring with an RAS-125c acoustic sensor, 15 out of 40 patients removed the capnography cannula, while only one removed the RAM acoustic sensor.3 In a study of 98 patients consciously sedated during upper gastrointestinal endoscopy, researchers found that RRa monitoring with the RAS-125c sensor more accurately assessed respiration rate than impedance pneumography.2
Subscribe to:
Posts (Atom)