Roth Capital analyst Yasmeen Rahimi reiterated a Buy rating and $30.00 price target
Search This Blog
Tuesday, July 2, 2019
Wright Medical down 7% on softer prospects for Cartiva implant
Wright Medical Group N.V. (WMGI -7.4%) slumps on more than double normal volume in response to “less positive” market feedback on its Cartiva synthetic cartilage implant designed to treat osteoarthritis in the big toe joint.
In a note, RBC Capital analyst Brandon Henry says some early adopting surgeons have reduced usage, adding that recent data appears to suggest that the device “may not be the panacea some initially hoped for.” He has lowered his fair value target to $34 from $36 while maintaining his Outperform rating.
Shares also sold off on June 20 after Wells Fargo said a number of doctors had temporarily stopped using Cartiva due to concerns with durability.
Medical Properties up 4% on bullish SunTrust call
Citing the potential for Medical Properties Trust (MPW +3.6%) to capitalize on its “large acquisition pipeline,” SunTrust’s Michael Lewis upgrades the stock to Buy with a $20 (11% upside) price target.
Mr. Lewis likes the upside from domestic deals considering the lower valuations.
Ritter up on advancement of RP-G28
Topical Probiotics for Atopic Dermatitis and Eczema
Atopic dermatitis is an inflammatory disease of the skin in which the normal barrier function of the skin is disrupted, so that immune function is impaired and susceptibility to infection with common bacteria like Staphylococcus aureus is increased.
This invasion by pathogenic microbes in turn leads to a disturbance in the numbers and relative proportions of various microbial species that live as commensal organisms on the skin, causing cutaneous dysbiosis.
Why is eczema treatment so urgent?
Atopic dermatitis is a common skin disorder, affecting about one in five children in developed nations. Moreover, it can profoundly disturb the individual’s quality of life and healthcare expenditures. Furthermore, atopic dermatitis is associated with development of asthma, allergic rhinitis, and food allergies. Children will often not cooperate with the multiple applications and oral medications that make up its treatment at present, and either way, their quality of life is decreased. More effective biologics are now available but the cost is still steep, putting it out of reach in many places.
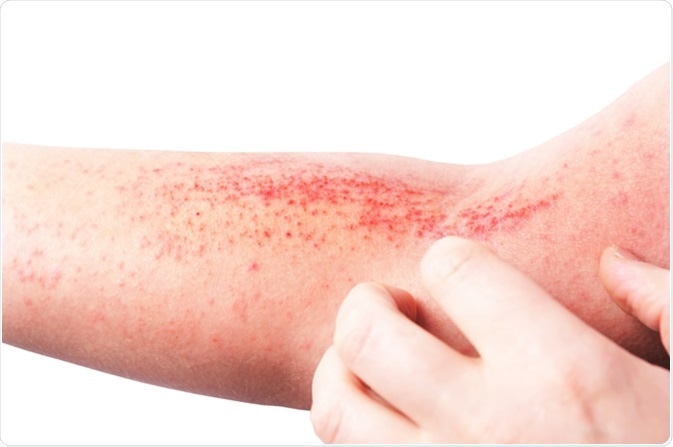
Atopic dermatitis (AD), also known as atopic eczema. Image Credit: LIAL / Shutterstock
Its incidence is shooting up in more developed nations, but most affected people will experience a marked reduction in symptoms around the time of puberty.
The chronic risks do not stop here, however, for the long duration during which the skin fails to provide a proper barrier to the invasion of foreign microbes and the removal of moisture takes its toll in the subsequent development of a hypersensitive skin which reacts to many other antigens.
How does the skin microbiota affect eczema?
Theoretically, topical and preventive oral antibiotics against S. aureus should be effective in treating atopic dermatitis. However, many studies have shown the reverse, so antimicrobial treatment are not part of standard treatment. It may be that there are more microbes to consider than just S. aureus (a gram-positive bacterium) in the pathogenesis of this disorder. Gram-negative bacteria on the skin are lower in skin areas most commonly affected by eczema compared to healthy persons.
Earlier studies have shown how important the skin microbiome is in eczema. Mapping the locations of skin Gram-negative bacteria revealed a stunning overlap with the sites where eczema typically breaks out – the antecubital fossa (front of the elbow) and the back of the knees, for instance. This suggests that when the wrong strains of certain bacteria replace the beneficial ones, atopic dermatitis may result. This led to scientists trying to correct the skin microbiome to treat eczema.
Spraying skin with bacteria successfully treats eczema
A recent experimental study used a Gram-negative commensal bacterium, Roseomonas mucosa, taken from the skin of healthy volunteers as well as from people with the condition. This microbe was studied on cell cultures and in mice.
Interestingly, in cell cultures R. mucosa killed S. aureus, while in mice it enhanced the skin’s barrier function, keeping it hydrated and preventing the entry of pathogens. Moreover, it also improved atopic dermatitis rashes in mice.
In a small human trial, the treatment improved the immune status of skin cells. However, the R. mucosa cultured from the skin of people with atopic dermatitis had a negative or no impact on the rashes.
During the first part of the trial, designed to look at the effectiveness and safety of the therapy, a mix of three live R. mucosa strains collected from healthy volunteers, dispersed in 10% -15% sucrose solution (sugar water), was sprayed on two skin sites, one in the front of the elbow and the other site chosen by the patient) by the adult subjects in preset increasing doses, from 103 to 105 colony-forming units (CFU) at each treatment site, twice a week for 6 weeks.
In the second part of the trial, five children were enrolled and treated with the same dose regimen for 12 weeks. From weeks 13-16, the treatment was escalated to once every alternate day.
Important findings
The findings of this trial, early though it is, were remarkable in some ways. For one, the administration of R. mucosa in mice was not associated with any toxicity, and there were no reported problems or adverse effects in humans either. The investigators found that both patient symptoms like itching and objective signs of the disease were significantly reduced. Steroid applications were decreased.
Secondly, the present trial achieved a treatment response beyond the minimum required effect level that is statistically significant for treatment effect, that is, >50% improvement in the SCORAD. This is in comparison to the historical placebo effect of 5% to 30%.
In children treated with topical probiotics, this level of improvement was seen in almost 80% of patients; 85% of treated adults also improved.
The overall number of patients who showed clinical improvement (68%) was greater than the 27% who would normally be expected to improve in accordance with the natural history of the disease.
All responders showed continued benefit even after the washout phase, with some manifesting additional improvement.
Strain-specific R. mucosal metabolites can promote dysbiosis in atopic dermatitis by decreased production of phospholipids which damage the epithelial barrier, compounds like histidinol, and monomethylgluterate, which irritate the epithelium, and reduce skin immunity. Thus providing topical “good” strains of R. mucosa could be of benefit to humans with atopic dermatitis in all these aspects.
The growth of beneficial R. mucosa is also affected by skin exposure to compounds like parabens which are commonly used as preservatives in soaps and skin products, but not that of S. aureus or R. mucosa from diseased skin. On the other hand, dilute bleach selectively inhibited S. aureus and R. mucosa from diseased individuals, but not from healthy volunteers.
Which patients are less likely to respond?
Responders were less likely than non-responders to have a family history of adult atopic dermatitis or at least three generations affected by atopic dermatitis.
Conclusion
This small preliminary study suggests several implications.
Different strains of R. mucosa produce different effects on the skin in patients with atopic dermatitis. When taken from normal volunteers with healthy skin, these organisms may produce clinically significant benefit in terms of improvement of skin rashes and itching, which is not seen when the organisms is isolated from the unaffected patches of skin in individuals with eczema.
The topical application of the bacteria may help restore the epithelial barrier function, correct the imbalance between innate and adaptive immunity, and restrict the growth of S. aureus leading to a lower population of this organism.
Genetic factors may also affect the response to treatment using R. mucosa.
Certain skin products may worsen atopic dermatitis by affecting the colonization of beneficial R. mucosa strains but not of pathogenic strains of S. aureus.
The next step will be to conduct a placebo-controlled trial for patients with atopic dermatitis to confirm or disprove these findings.
Sources
- Myles I. A. et al., (2018). First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight. https://doi.org/10.1172/jci.insight.120608
- Shoup M. E. (2018). Topical Probiotics for Atopic Dermatitis and Eczema. www.nutraingredients-usa.com/…/Topical-microbiome-transplant-may-improve-atopic-dermatitis-Study
Weight loss support helps people with fatty liver disease
People who have fatty liver disease related to being overweight may get the disease under better control when they get lots of support to lose weight, a research review suggests.
Most people have a little bit of fat in their liver, but fatty liver disease can be diagnosed when more than 5% of the liver is made up of fat. If the condition isn’t linked to liver damage from heavy drinking, it’s known as non-alcoholic fatty liver disease (NAFLD) and is most often associated with obesity and certain eating habits.
For the current analysis, researchers examined data on 2,588 patients who were participating in 22 clinical trials of various interventions to help them lose weight. Fifteen studies tested behavioral weight loss programs; six tested medications; one tested weight loss surgery.
The trials also looked at whether those interventions would improve biomarkers for NAFLD that can help predict the likelihood of serious complications.
Compared with little or no weight loss support, the interventions that offered the most support were associated with greater weight loss and bigger reductions in biomarkers for NAFLD like elevated liver enzymes in the blood, elevated blood sugar, and reduced sensitivity to the hormone insulin, or insulin resistance.
“It shows clearly that weight loss improves the health of the liver,” said Dimitrios Koutoukidis, a researcher at the University of Oxford in the U.K. and lead author of the study.
“We found some evidence that weight loss improved NAFLD through improvements in the control of blood glucose levels and reductions in insulin resistance, but we need more research to understand the exact mechanisms,” Koutoukidis said by email.
Different approaches to weight loss didn’t appear to impact whether fibrosis, or scarring, in the liver got better or worse.
Worldwide, about one in four adults have NAFLD, as do at least half of people with obesity, researchers note in JAMA Internal Medicine.
There’s no drug treatment for NAFLD. Doctors typically advise patients to lose weight by cutting calories and getting more exercise, or sometimes by taking weight-loss medications or considering weight-loss surgery. The new findings, according to the researchers, “appear to support the need to change the clinical guidelines and to recommend formal weight loss programs for people with NAFLD.”
One limitation of the analysis is that the smaller studies tested a wide range of weight-loss interventions over varying lengths of time and used different tests to assess patients’ liver disease.
Still, weight loss through a combination of dietary improvements and increased exercise can improve fatty liver, said Dr. Danielle Brandman, director of the Fatty Liver Clinic at the University of California San Francisco and coauthor of an editorial accompanying the study.
Ideally, patients should try to lose 7% of their weight and maintain this weight loss in order to have long-term improvements in NAFLD, Brandman said by email.
“Patients have the potential to improve or cure their disease,” Brandman said.
“However, they should know that this is an ultra-marathon rather than a sprint,” Brandman added. “Weight loss – and the behavior change needed to achieve it – can be really difficult for many patients for a variety of reasons.”
SOURCE: bit.ly/2ZZq9Cs JAMA Internal Medicine, online July 1, 2019.
Vietnam says will have African swine fever vaccine ‘soon’, experts skeptical
Vietnam said on Tuesday it has had initial success in creating a vaccine to fight African swine fever, which has infected farms throughout the Southeast Asian country and prompted the culling of around 10% of its pig herd.
African swine fever – which has spread to Laos and North Korea as well after being detected in China in August 2018 – was first detected in Vietnam in February and has spread to farms in 61 of the country’s 63 provinces.
More than 2.9 million pigs have been culled in Vietnam, Agriculture Minister Nguyen Xuan Cuong said on Tuesday, out of a hog population of about 30 million.
“I think we’re on the right track, and we will soon have a vaccine,” Cuong said, according to the official Vietnam News Agency (VNA).
The vaccine, developed at the Vietnam National University of Agriculture, has been tested in its laboratory and at three farms in northern Vietnam, state broadcaster Vietnam Television (VTV) said in a separate report on Tuesday.
Experts on vaccines and African swine fever, though, were skeptical over the claims of progress and said there needed to be much more research to prove the viability of any vaccine.
“We need different phases of clinical trials, first in an experimental setting with controlled exposure, and then a field trial with natural exposure to the virus, and that cannot be a small trial,” said Dirk Pfeiffer, a professor of veterinary epidemiology at the City University of Hong Kong.
The complex nature of the virus and gaps in knowledge concerning infection and immunity have so far hindered other global efforts to develop a vaccine against the disease, which is harmless to humans but deadly to pigs.
Researchers elsewhere have abandoned attempts to use a killed virus for a vaccine and teams in the United States, Europe and China have been working on live vaccines instead, which carry higher safety risks.
In Vietnam’s initial trials, 31 out of 33 pigs injected with the test vaccine are still healthy after receiving two shots over a period of months, according to the VTV report.
Other pigs at the farms have died from the virus, the report said, without giving specific figures. No further details were given about the vaccine or the trials.
The agricultural university’s director, Nguyen Thi Lan, said the vaccine still needed further research, and required testing on a larger scale.
Lan declined to comment on the report and referred questions from Reuters to the agricultural ministry, which did not immediately respond to a request for comment.
Pork makes up three-quarters of total meat consumption in Vietnam, a country of 95 million people where most of its farm-raised pigs are consumed domestically.
The country’s pork industry is valued at 94 trillion dong ($4 billion) a year, and accounts for nearly 10% of Vietnam’s agricultural sector.
African swine fever was first detected in Asia last year in China, the world’s largest pork producer. As many as half of China’s breeding pigs have died or been slaughtered because of the disease, twice as many as officially reported.
Subscribe to:
Comments (Atom)