The U.S. Department of Veterans Affairs and Apple collaborated to provide veterans easier access their medical information even if they seek care from multiple providers. (Apple)
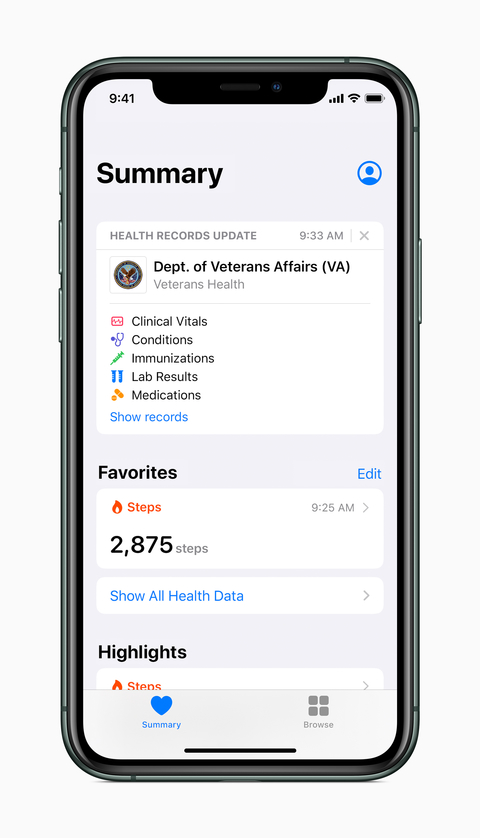
The U.S. Department of Veterans Affairs (VA) and Apple announced Wednesday that millions of U.S. veterans will now be able to access their medical records on their iPhones through the tech giant’s health records feature.
The VA has been collaborating with Apple for months to roll out the new health record app after first announcing the initiative back in February. The app makes it easier for veterans to access their medical data across multiple providers by combining their VA health data alongside their health records from community providers, Apple and VA officials said.
The VA is the largest medical system in the U.S., providing service to more than 9 million veterans across 1,243 facilities, including hospitals and clinics.
The VA joins Johns Hopkins, University of California San Diego, Quest Diagnostics, Allscripts and 400 other health care provider organizations, laboratory networks, and electronic health records (EHRs) vendors who support the health records on iPhone feature, Apple executive said in a blog post.
The VA plans to partner with other organizations to bring similar capabilities to other mobile platforms, according to a VA press release.
“Helping veterans gain a better understanding of their health is our chance to show our gratitude for their service,” Jeff Williams, Apple’s chief operating officer, said in a blog post. “By working with the VA to offer Health Records on iPhone, we hope to help those who served have greater peace of mind that their health care is in good hands.”
We’re teaming up with the Department of Veterans Affairs to ensure our nation’s heroes have consistent and secure access to their medical data through the Health app. https://t.co/t2aDymbCJd— Tim Cook (@tim_cook) November 6, 2019
Through the Health Records for iPhone app, veterans can see all of their health records in one place, including medications, immunizations and lab results. The app continually updates the records, giving VA patients access to a single, integrated snapshot of their health profile, VA and Apple officials said.
Aneesh Chopra, former White House chief technology officer under the Obama administration and president of healthcare company CareJourney, applauded the initiative in a tweet, saying its part of an ongoing effort to open up patient access to health information.
Another benefit of this move is to ease the implementation burden on the @DeptVetAffairs MISSION Act for veteran access to community care; a veteran can aggregate his or her records today across many systems!consumer-mediated exchange!@carinalliance https://t.co/aLrkyCX0UV— Aneesh Chopra (@aneeshchopra) November 6, 2019
“We have delivered veterans an innovative new way to easily and securely access their health information,” VA Secretary Robert Wilkie said in a statement. “Veterans deserve access to their health data at any time and in one place, and with Health Records on the Health app, VA has pushed the Veterans experience forward.”
This capability was developed through the VA’s Veterans Health Application Programming Interface (Veterans Health API). The VA gradually launched Health Records to select patients this summer and the service now tops 2,000 users, according to the VA.
The Veterans Health API allows private sector organizations to create and deploy digital applications that help veterans access their health records in new ways, the VA said.