1968 was a bad year for flu but, as pandemics go, it was pretty mild.
Scientists called the flu strain that hit the world H3N2. It’s still
around.
Globally, about one million people died until the outbreak faded
during the winter of 1969-70. In the U.S., the death toll was
approximately 100,000 – three or four times the average annual death
toll for flu since 2010, according to CDC figures. Most of those deaths were among people age 65 or older.
Although the COVID-19 death toll has not yet reached the numbers of
H3N2, it still continues to climb. According to Johns Hopkins
University’s continually updated Coronavirus Resource Center, global deaths for COVID-19 are now in excess of 116,000, and the U.S. has seen over 22,000.
The H3N2 flu originated in Hong Kong in July 1968, appeared in the
U.S. in September and is still circulating as a type of Influenza A.
H3N2 was present among the 2019 flu strains and in the swine flu outbreak earlier in the decade.
Like so many viruses implicated in 20th century pandemics, both the
H3N2 virus and the SARS-Cov-2 virus that causes COVID-19 exhibited
cross-species transmission, appearing first in animals before jumping to
humans and, sometimes, back to animals. A canine outbreak occurred in late 2017 in Ontario, Canada and persisted until October 2018.
H3N2 is considered one of the most troubling flu strains because, like COVID-19, it is highly contagious.
Scientists suspect that H3N2 emerged through an antigenic shift, in
which the hemagglutinin (H) H2 antigen on the surface of the virus
mutated to become the H3 antigen. According to the CDC, “The H3N2 virus
is comprised of two genes from an avian influenza A virus, including a
new H3 hemagglutinin, but also contains the N2 neuraminidase from the
1957 H2N2 virus.”
When sequencing the SARS-CoV-2 genome
in January, researchers found it encodes a large, non-structural
polyprotein that is proteolytically cleaved to generate additional
proteins, including four structural proteins (the spike surface
glycoprotein, the membrane, envelope and nucleocapsid protein) and five
accessory proteins.
The small changes that characterize antigenic drift generally result
in viruses that are only minimally different, so a vaccine designed for
one usually is effective for a slightly mutated version.
With the seasonal flus, like H3N2, antigenic drift is continual. The
accumulated effects of antigenic drift, however, can result in viruses
that are so different from the original virus that the immune system
doesn’t recognize them. Whether it will play a role in COVID-19 is still
unknown.
Because H3N2 was closely related to the 1957 pandemic, many people
were immune. This kept the 1968 H3N2 flu epidemic relatively mild,
especially when compared to the 1918 Spanish flu. For some reason,
however – possibly antigenic drift – the second wave of the H3N2 flu
that struck in 1969 was more deadly.
Differences in immunity are evident as the virus mutated during its
global spread, as shown by the different patterns of infection and
death.
Edwin D. Kilbourne, (since deceased) professor of microbiology and immunology at New York Medical College, in a 2006 article
comparing the influenza pandemics of the 20th century, found that
outbreaks in Japan were small and scattered. Western Europe, including
the United Kingdom, experienced increased illnesses but no increased
death rates during the first year of the pandemic. The U.S., however,
experienced high illness and death rates the first year, beginning on
the West Coast where the virus was first introduced.
“Researchers speculated that (H3N2’s) more sporadic and variable
impact in different regions of the world were mediated by differences in
prior N2 immunity. Therefore, the 1968 pandemic has been aptly
characterized as ‘smoldering,’” Kilbourne wrote. Emphasizing that point,
vaccination by Air Force cadets with the H2N2 vaccine reduced H3N2 infections by 54%.
In the current pandemic, patterns of infection and mortality are
fairly consistent throughout the world. The major mitigating influence
is the degree of social distancing and the presence of comorbidities.
The elderly are at high risk of infection but, unlike H3N2, not the very
young.
In the 1960s, vaccines were evolving. The 1968 N3N2 pandemic triggered
the development of trivalent vaccines and of subunit vaccines, which
decreased adverse reactions. About the same time, the U.S. began
recommending annual flu vaccination for high risk individuals.
Today, researchers are working to develop a vaccine for COVID-19
patients. ClinicalTrials.gov reported 28 vaccines in various stages of
trials throughout the world on April 10. The World Health Organization’s International Clinical Trials Registry Platform listed an additional 8. Availability is expected in 12 to 18 months, possibly in time for next year’s flu season.
“No one knows whether the virus (COVID-19) will disappear ultimately,
or will it persist like flu and become prevalent intermittently, will
it be like hepatitis B that resides in people without sufficient
immunity and spreads to others in that way?” said Wang Chen, the dean of
Peking Union Medical College, in an interview with Chinese News Agency
Xinhua.
Immunity, however, is uncertain. A growing number of cases are
presenting in which patients tested negative for SARS-CoV-2 virus
multiple times only to then test positive.
https://www.biospace.com/article/the-1968-pandemic-strain-h3n2-persists-will-covid-19-/
Search This Blog
Monday, April 13, 2020
FDA Approves Rutgers’ Saliva Test for COVID-19
Currently, the tests to diagnose COVID-19 require a swab from deep in the nasal passages or the back of the throat. The U.S. Food and Drug Administration (FDA) granted emergency use authorization (EUA) to Rutgers’ RUCDR Infinite Biologics
for its test that uses saliva. The test method was developed by RUCDR
with Spectrum Solutions and Accurate Diagnostic Labs (ADL).
“The impact of this approval is significant,” said Andrew Brooks, chief operating officer and director of technology development at RUCDR. “It means we no longer have to put health care professionals at risk for infection by performing nasopharyngeal or oropharyngeal collections. We can preserve precious personal protective equipment for use in patient care instead of testing. We can significantly increase the number of people tested each and every day as self-collection of saliva is quicker and more scalable than swab collections. All of this combined will have a tremendous impact on testing in New Jersey and across the United States.”
The Trump administration’s COVID-19 testing task force has already contacted RUCDR about how they can help expand testing and allow labs to leverage the technology. Biopharma executives for companies involved in COVID-19 testing also contacted the group.
“I have spoken with these companies’ leadership to not only share knowledge but to create opportunities for continuing to help innovate during this crisis,” Brooks said. ‘We will work closely with these new partners, the FDA and the White House task force to leverage everything Rutgers has to offer to not only help our community but also make a global impact.”
The COVID-19 pandemic has caused a massive focus on the part of life science companies to develop diagnostic tests for the virus as well as for serological antibody tests to determine if people have been exposed and have immunity to the disease.
On April 3, the FDA approved the first blood test for antibodies against COVID-19. That was by Research Triangle Park, North Carolina-based Cellex. About the same time, Mayo Clinic developed its own antibody test and began offering it on April 6.
Stanford Medicine researchers have joined the list of organizations that have developed an antibody test, which was launched April 6 at Stanford Health Care. It is different from an externally developed test Stanford utilized for a prevalence study during a community screening event.
The Stanford test provides results in two to three days and Stanford Health Care can test 500 samples per day. It believes it can scale that up quickly.
Although there are numerous vaccines in development, most optimistic projections don’t have one being available until mid- to late-2021 or possibly even later. Without a vaccine, the most likely exit from the pandemic will require wide-scale testing for the disease and antibodies against the disease followed by contact tracing—identifying who people who test positive for the disease have been in contact with and isolating them. People who test positive for antibodies against the virus will presumably have immunity and can proceed normally. At least, that’s how it usually works, but there is no evidence yet exactly how long such immunity would last.
This Rutgers test would definitely make the testing for the disease easier.
Jay A. Tischfield, founder, chief executive officer and scientific director of RUCDR said, “The test can help hospital-based and private physicians to accurately assess the infection status of more patients, with RUCDR Infinite Biologics doing the analysis.”
Brooks added, “Saliva testing will help with the global shortage of swabs for sampling and increase testing of patients, and it will not require health care professionals to be put at risk to collect samples. Saliva testing will also be important for people who are in quarantine because they don’t know how long it will be until they are no longer infectious. This will allow health care workers to release themselves from quarantine and safely come back to work.”
RUCDR recently announced it had launched a genetic testing service for SARS-CoV-2 that can test thousands of samples daily. The new saliva test has the potential to increase the numbers to tens of thousands of daily samples.
https://www.biospace.com/article/rutgers-s-saliva-test-for-covid-19-approved-by-fda/
“The impact of this approval is significant,” said Andrew Brooks, chief operating officer and director of technology development at RUCDR. “It means we no longer have to put health care professionals at risk for infection by performing nasopharyngeal or oropharyngeal collections. We can preserve precious personal protective equipment for use in patient care instead of testing. We can significantly increase the number of people tested each and every day as self-collection of saliva is quicker and more scalable than swab collections. All of this combined will have a tremendous impact on testing in New Jersey and across the United States.”
The Trump administration’s COVID-19 testing task force has already contacted RUCDR about how they can help expand testing and allow labs to leverage the technology. Biopharma executives for companies involved in COVID-19 testing also contacted the group.
“I have spoken with these companies’ leadership to not only share knowledge but to create opportunities for continuing to help innovate during this crisis,” Brooks said. ‘We will work closely with these new partners, the FDA and the White House task force to leverage everything Rutgers has to offer to not only help our community but also make a global impact.”
The COVID-19 pandemic has caused a massive focus on the part of life science companies to develop diagnostic tests for the virus as well as for serological antibody tests to determine if people have been exposed and have immunity to the disease.
On April 3, the FDA approved the first blood test for antibodies against COVID-19. That was by Research Triangle Park, North Carolina-based Cellex. About the same time, Mayo Clinic developed its own antibody test and began offering it on April 6.
Stanford Medicine researchers have joined the list of organizations that have developed an antibody test, which was launched April 6 at Stanford Health Care. It is different from an externally developed test Stanford utilized for a prevalence study during a community screening event.
The Stanford test provides results in two to three days and Stanford Health Care can test 500 samples per day. It believes it can scale that up quickly.
Although there are numerous vaccines in development, most optimistic projections don’t have one being available until mid- to late-2021 or possibly even later. Without a vaccine, the most likely exit from the pandemic will require wide-scale testing for the disease and antibodies against the disease followed by contact tracing—identifying who people who test positive for the disease have been in contact with and isolating them. People who test positive for antibodies against the virus will presumably have immunity and can proceed normally. At least, that’s how it usually works, but there is no evidence yet exactly how long such immunity would last.
This Rutgers test would definitely make the testing for the disease easier.
Jay A. Tischfield, founder, chief executive officer and scientific director of RUCDR said, “The test can help hospital-based and private physicians to accurately assess the infection status of more patients, with RUCDR Infinite Biologics doing the analysis.”
Brooks added, “Saliva testing will help with the global shortage of swabs for sampling and increase testing of patients, and it will not require health care professionals to be put at risk to collect samples. Saliva testing will also be important for people who are in quarantine because they don’t know how long it will be until they are no longer infectious. This will allow health care workers to release themselves from quarantine and safely come back to work.”
RUCDR recently announced it had launched a genetic testing service for SARS-CoV-2 that can test thousands of samples daily. The new saliva test has the potential to increase the numbers to tens of thousands of daily samples.
https://www.biospace.com/article/rutgers-s-saliva-test-for-covid-19-approved-by-fda/
China Obstructs Hunt For COVID-19 Cure By Canceling ‘Promising’ Gilead Study
As China continues its push to conceal and crack down on
coronavirus-related research happening inside its borders, Gilead’s CEO
reported over the weekend that Beijing had shut down a branch of its
closely watched global remdesivir that was studying patients in ‘severe’
condition in Wuhan. After showing early promise, the study was
allegedly shuttered by the government because there weren’t enough
patients who qualified.
Beijing would love it if the world truly believed that there aren’t anymore COVID-19 patients in Wuhan who are still in serious condition. But even as the city opens up (with Beijing promising Monday to institute stricter checks on people attempting to leave the city), suspicions about China undercounting cases make this claim extremely hard to believe.
So, why did China shut down a study where preliminary data appeared to show progress in healing moderately sick patients in the West? It’s almost as if they don’t want the rest of the world to develop a treatment.
In an open letter published over the weekend, Gilead CEO Daniel O’Day
argued that more data from the global drug trial should be available by
the end of April. However, he noted Chinese authorities who stopped the
trial in Wuhan haven’t seen when they plan to turn over Gilead’s data.
In his letter, O’Day made the cancellation seem like no big deal. We suspect that framing was intended to placate Beijing.
Still, CNBC was still touting on Monday results from a different branch of preliminary remdesivir study in the West being supervised by Gilead. If this data are still so promising, why would China shut this trial down? The decision will likely delay the start of the next, more intensive, phase of clinical trials with control groups allowing for more definitive data, even as a more intensive study at the NIH in the US continues, with talk of ‘approval’ as early as May.
Referring to the preliminary results cited last week,
analysts at RBC said “the data are likely not sufficient to convince
people that this could substantially end the crisis, but enough to
believe there is some chance remdesivir, along with other meds in
development, could play a potential role helping somewhat blunt
morbidity/mortality.”
But why would Beijing want to block the US from developing an effective treatment for COVID-19? If party officials truly want the world to stop blaming them for the outbreak, shouldn’t they be doing everything they can to help the world find a cure?
We’ll let you figure that one out.
https://www.zerohedge.com/geopolitical/china-obstructs-hunt-coronavirus-cure-canceling-promising-gilead-study
Beijing would love it if the world truly believed that there aren’t anymore COVID-19 patients in Wuhan who are still in serious condition. But even as the city opens up (with Beijing promising Monday to institute stricter checks on people attempting to leave the city), suspicions about China undercounting cases make this claim extremely hard to believe.
So, why did China shut down a study where preliminary data appeared to show progress in healing moderately sick patients in the West? It’s almost as if they don’t want the rest of the world to develop a treatment.
We know that there is tremendous interest around when the data from these trials will be available and what they will tell us about remdesivir. We feel the urgency as we wait for the science to speak. With every day that goes by, the desperate need to equip healthcare workers and their patients with a safe, effective treatment becomes more pressing. We are working with intense speed to determine whether remdesivir could be an option and we are committed to sharing information when it becomes available to us.Wuhan has been conducting the research in Wuhan for more than 2 months.
We expect that we will have preliminary data from the study of remdesivir in severe patients at the end of April and will work quickly to interpret and share the findings. The publication of data from the China remdesivir trials rests with the Chinese investigators, but we have been informed that the study in patients with severe symptoms was stopped due to stalled enrollment. We look forward to reviewing the published data when available. In May, we anticipate the initial data from the placebo-controlled NIAID trial as well as data from the Gilead study of patients with moderate symptoms of COVID-19.
In his letter, O’Day made the cancellation seem like no big deal. We suspect that framing was intended to placate Beijing.
Still, CNBC was still touting on Monday results from a different branch of preliminary remdesivir study in the West being supervised by Gilead. If this data are still so promising, why would China shut this trial down? The decision will likely delay the start of the next, more intensive, phase of clinical trials with control groups allowing for more definitive data, even as a more intensive study at the NIH in the US continues, with talk of ‘approval’ as early as May.
But why would Beijing want to block the US from developing an effective treatment for COVID-19? If party officials truly want the world to stop blaming them for the outbreak, shouldn’t they be doing everything they can to help the world find a cure?
We’ll let you figure that one out.
https://www.zerohedge.com/geopolitical/china-obstructs-hunt-coronavirus-cure-canceling-promising-gilead-study
Morgan Stanley Publishes Full Timeline Of Upcoming Coronavirus Milestones
Sees Second Coronavirus Peak In December
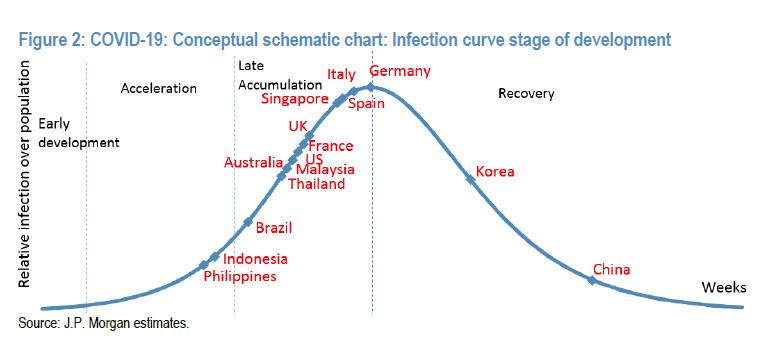
Now that it has become clear that every day that the US economic shutdown continues as a result of the coronavirus pandemic means billions in economic losses and untold damages to the social fabric of the United States where over 20 million people will soon be unemployed, what all analysts – and frankly everyone else – want to know is i) when will the US reach the peak of the coronavirus curve and ii) when will the US start reopening.Addressing the first, we showed some good news yesterday when the latest JPMorgan coronavirus “curve” showed the US fast approaching the peak of the curve, i.e., the end of the “late accumulation” phase, and sliding into recovery.
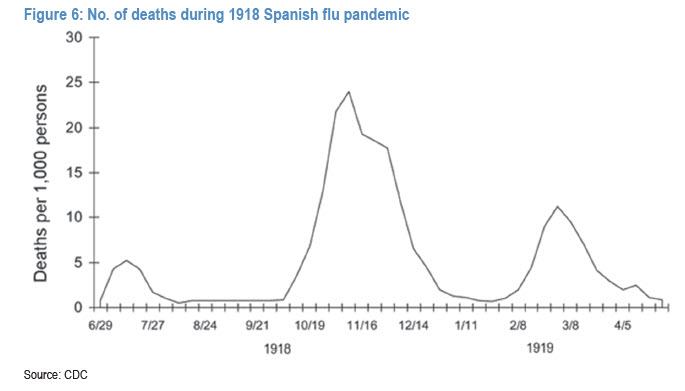
Yet while the first wave of the pandemic appears to be approaching its zenith, the big concern is that a second, even more powerful wave may emerge afterwards if the Spanish flu pandemic is any indication.
Unfortunately validating fears that the first wave is only the beginning, in a Sunday note from Morgan Stanley’s chief biotech analyst Matthew Harrison, he writes that “recovering from this acute period in the outbreak is just the beginning and not the end” and “the path to re-opening the economy is going to be long. It will require turning on and off various forms of social distancing and will only come to an end when vaccines are available, in the spring of 2021 at the earliest.”
Specifically, the biotech strategist expects a “multiphasic” peak in which coastal regions, led by New York, will peak “over the next 3-5 days. However, we expect the rest of the country to follow slowly, trailing the coasts by around three weeks.” And while this “second” peak is unlikely to be as severe as the first (~10,000-15,000 daily new cases versus 30,000-35,000 in the first peak), “it means the US outbreak will have a very long tail. This much longer tail would put the US time to peak at ~4x China and 2x Italy, driven by the slow uptake of social distancing measures and lack of robust testing. This would put an initial US reopening on track for mid-to-late May at the earliest.”
It gets worse, with the biotech analyst also predicting that there “will still be a large number of workers not able to go back to work until a vaccine is abundantly available as social distancing cannot be fully relaxed until we have herd immunity (~60% of people vaccinated). Furthermore, large venues such as sports stadiums, concert halls and theme parks are also likely to remain shut or have attendance capped at 10-25% of prior levels.”
Finally, and most ominous, Morgan Stanley warns that a potential second wave of infections could strike around November/December, which the bank hopes will be less severe than the current peak, although in truth nobody knows.
“This view on the delayed peak and slow return to work has led our US economists to revise their US forecast to a return to pre-COVID-19 levels not until 4Q21” Harrison writes, but concludes on a positive note, pointing out that there are promising antivirals and antibody therapies in the pipeline with data starting in April and continuing through the late summer.
We believe that at least some of these drugs can be successful and help to turn severe cases into milder forms of the disease. Such an outcome could reduce the potential strain on hospitals and allow public health officials to support a broader re-opening of the economy before a vaccine is availableOne can only hope that he is right.
Below is the full timeline of upcoming key coronavirus milestones from Morgan Stanley:
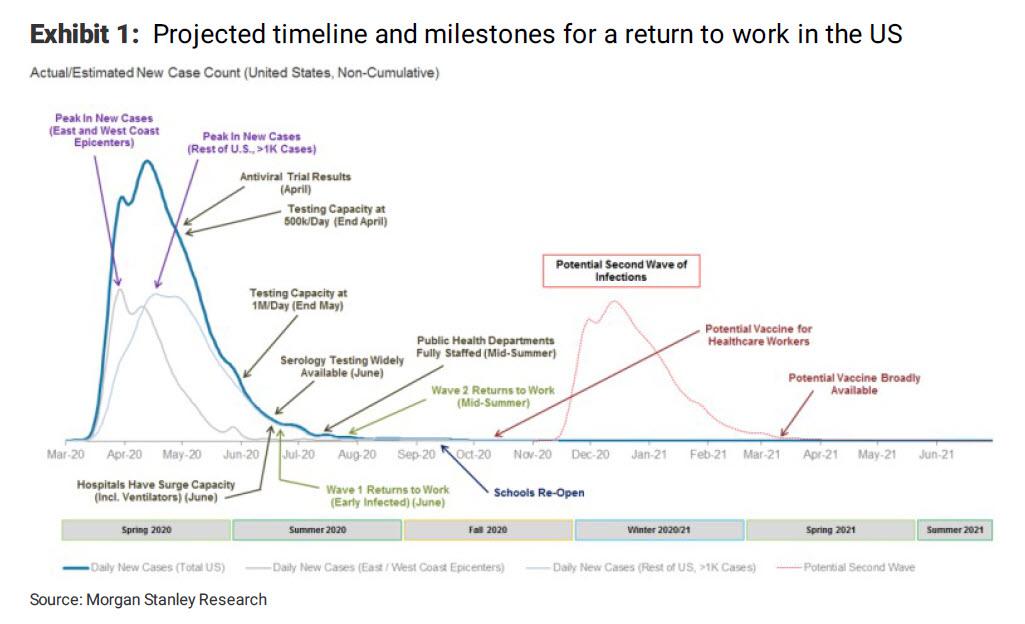
* * *
His full note is below:
Welcome to your first and only chance to have a Morgan Stanley biotech analyst tell you what he thinks about the market. Over the past week it has been striking to see how investors have reacted to the first signs that new COVID-19 cases in New York are starting to stabilize. While we understand the desire for optimism, we also caution that the US outbreak is far from over. Recovering from this acute period in the outbreak is just the beginning and not the end. We believe the path to re-opening the economy is going to be long. It will require turning on and off various forms of social distancing and will only come to an end when vaccines are available, in the spring of 2021 at the earliest.https://www.zerohedge.com/health/morgan-stanley-publishes-full-timeline-upcoming-coronavirus-milestones-sees-second
With Italy finally turning the corner in its outbreak as the growth in new daily cases has started to decline, the market has turned its attention to the US. We recently completed a state-level model for the US which suggests it is likely to face a multiphasic peak. In particular, we expect the coastal regions, led by New York, to peak – defined as a sustained decline in new daily cases – over the next 3-5 days. However, we expect the rest of the country to follow slowly, trailing the coasts by around three weeks. While this “second” peak is unlikely to be as severe as the first (~10,000-15,000 daily new cases versus 30,000-35,000 in the first peak), it means the US outbreak will have a very long tail. This much longer tail would put the US time to peak at ~4x China and 2x Italy, driven by the slow uptake of social distancing measures and lack of robust testing (New York, with the highest testing ratio in the US, is still testing at a per capita rate just half that of South Korea’s most impacted city Daegu). This would put an initial US reopening on track for mid-to-late May at the earliest.
Investors should realize that this won’t be a “normal” re-opening. In COVID-19: A Prescription to Get the US Back to Work, we argue that only after we see (1) adequate surge capacity in hospitals, (2) broad public health infrastructure to support testing for disease surveillance, (3) robust contact tracing to curtail “hot spots” and (4) widespread availability of serology testing (blood tests to see who is already immune to the virus) can the US confidently return to work. We see this happening in waves starting in mid-summer. Unfortunately, we think there will still be a large number of workers not able to go back to work until a vaccine is abundantly available as social distancing cannot be fully relaxed until we have herd immunity (~60% of people vaccinated). Furthermore, large venues such as sports stadiums, concert halls and theme parks are also likely to remain shut or have attendance capped at 10-25% of prior levels. This view on the delayed peak and slow return to work has led our US economists to revise their US forecast to a return to pre-COVID-19 levels not until 4Q21.
Despite the significant concerns we raise about the path to a US recovery, we continue to believe the market is underestimating the impact the drug pipeline can have on the public policy response to the virus. We should stress that investors cannot afford to lose sight of the fact that only a vaccine will provide a true solution to this pandemic. We also believe that governments should invest in large-scale vaccine manufacturing prior to successful results for all viable candidates despite some not making it to market. Only by building production capacity now can governments provide the billions of vaccine doses we need to meet demand for the 2021 season. That said, in the interim there are promising antivirals and antibody therapies in the pipeline with data starting in April and continuing through the late summer. We believe that at least some of these drugs can be successful and help to turn severe cases into milder forms of the disease. Such an outcome could reduce the potential strain on hospitals and allow public health officials to support a broader re-opening of the economy before a vaccine is available. Thus, with therapeutics available in the near term and a vaccine on the horizon, the market could start to “look through” the slow US recovery and back to pricing in future US growth.
Carnival’s stock dives after cruises cancelled through June 26
Shares of Carnival Corp. CCL, -8.87%
took an 10.1% dive in morning trading Monday, after the cruise operator
said it has cancelled all North American cruise itineraries through
June 26. The company said it also cancelled all Carnival Sunrise
itineraries out of New York to the end of this year. “This is
disappointing, but we are committed to being a strong partner with the
government and taking steps to maintain public confidence in our
business,” Carnival said in a statement released in a tweet.
Late last week, the Centers for Disease Control and Prevention extended
the No Sail Order for cruise ships for roughly three months in response
to the COVID-19 pandemic. Among other cruise operators, shares of Royal
Caribbean Cruises Ltd. RCL, -14.29% plunged 15.0% and Norwegian Cruise Line Holdings Ltd. NCLH, -13.50%
slumped 14.2%. Carnival’s stock had rocketed 46.3% last week, the
best-ever weekly performance. the stock has now plummeted 77.4% year to
date, while the S&P 500 SPX, -1.62% has dropped 17.2%.
https://www.marketwatch.com/story/carnivals-stock-dives-after-cruises-cancelled-through-june-26-in-wake-of-cdcs-extended-no-sail-order-2020-04-13
https://www.marketwatch.com/story/carnivals-stock-dives-after-cruises-cancelled-through-june-26-in-wake-of-cdcs-extended-no-sail-order-2020-04-13
Pluristem Therapeutics Treats First US COVID-19 Patient
Pluristem Therapeutics Inc. (PSTI) said it has treated its first
patient suffering from COVID-19 under the FDA Single Patient Expanded
Access Program. Pluristem’s main target is to initiate a multinational
clinical trial as soon as possible. The company noted that it does not
intend to provide further updates on the status of patients treated
under compassionate use.
“We expect to continue treating patients under compassionate use through the appropriate regulatory clearances in the United States and Israel, as well as expanding treatment under compassionate use in other countries. Our main focus remains however, the initiation of a multinational clinical study,” said CEO Yaky Yanay.
https://www.nasdaq.com/articles/pluristem-therapeutics-treats-first-covid-19-patient-in-u.s.-2020-04-13
“We expect to continue treating patients under compassionate use through the appropriate regulatory clearances in the United States and Israel, as well as expanding treatment under compassionate use in other countries. Our main focus remains however, the initiation of a multinational clinical study,” said CEO Yaky Yanay.
https://www.nasdaq.com/articles/pluristem-therapeutics-treats-first-covid-19-patient-in-u.s.-2020-04-13
Amazon to hire another 75K
It was about a month ago when the company announced plans to hire another 100K folks for full- and part-time positions.
That pledge having been filled, Amazon (NASDAQ:AMZN) this morning says it’s going to be hiring another 75K.
Also, a previous promise to spend $350M on wage increases has been boosted to north of $500M.
The move to online shopping now moving at warp
speed, Amazon is struggling to meet demand. Deliveries are being delayed
and/or lost, and the company today said it wasn’t – for now – accepting new online grocery
customers. It’s shortening open hours at Whole Foods so employees can
instead spend time trying to take care of delivery orders.
With the broad market in the red this session, Amazon shares up are up 3%.
https://seekingalpha.com/news/3560164-amazon-to-hire-another-75k
Subscribe to:
Comments (Atom)