If you’re thinking about visiting a museum these days, you probably care about health and safety protocols like mask wearing, readily available hand sanitizer, temperature checks, and reduced capacity.
But the very air itself—and the way it circulates—is a huge factor in the coronavirus pandemic.
That’s where heating, ventilation, and air conditioning systems (HVAC) come into play, reducing the concentration of viral particles in the air and making it less likely that visitors will be exposed to airborne germs.
“Of all the measures that LACMA is implementing, HVAC is probably the most critical after social distancing and face covering,” a Los Angeles County Museum of Art spokesperson told Artnet News in an email.
Before US museums closed last March, many institutions began implementing “rigorous cleaning protocols,” putting sanitation workers into overdrive to eradicate potential fomites from high-touch surfaces.
But as scientists began to better understand the novel coronavirus and how it was transmitted, it became clear that what we should really be worried about was airborne transmission.
“Appropriate ventilation can help reduce the concentration of viral particles in indoor air. Conversely, poorly designed airflow can actually increase risk of transmission,” the American Alliance of Museums explained in a guide issued after the onset of the pandemic.
Fortunately, museums are held to pretty high air-quality standards by the American Society of Heating, Refrigerating, and Air-Conditioning Engineers, or ASHRAE, to maintain optimal conservation conditions.
“For preservation purposes with the art, there’s a very, very stringent indoor requirement in terms of temperature and humidity that needs to be maintained,” said Molly Dee, head of deep carbon reduction at the consulting firm Jaros, Baum & Bolles, which worked on the 2019 expansion of the Museum of Modern Art, and helped survey the facilities ahead of its reopening.
“The design of our air systems, which have high performance filters in place to meet preservation needs, also helps us adhere to current ventilation guidelines,” a representative of the Fine Arts Museums of San Francisco told Artnet News in an email. “We always monitor environmental conditions, especially temperature and relative humidity, closely and continuously. This allows us to detect the impact of any changes, and to make adjustments to artwork displays as needed.”
Across town, the San Francisco Museum of Modern Art is frequently pressed into service as a “breathing center” during California fire season. The museum’s lobby and public spaces offer fresh, clean air to the public when the state’s air quality hits unsafe levels.
And at the Museum in Fine Arts in Houston, the first major museum in the country to reopen, a high-tech ventilation system was always especially important because of the local climate. (The museum installed new HVAC systems in two of its three buildings only three years ago.)
“Because of the problems with humidity and the possibility of mold growth, which is pervasive in our atmosphere, ventilation is really important,” director Gary Tinterow told Artnet News. “What’s good for works of art is good for human beings also. Museum environments are exceptionally clean and healthy.”
In its official ventilation in buildings guidelines, the CDC does not recommend that businesses replace their ventilation systems to protect against COVID, but does advise that “upgrades or improvements can increase the delivery of clean air and dilute potential contaminants.”
For some museums, those improvements include increasing the frequency with which air circulates through the building.

The Museum of Contemporary Art Chicago. Photo by Peter McCullough, ©MCA Chicago.
At the MFAH, “in any given space the air is going to change every five to seven minutes,” Tinterow said. “We increased the amount of fresh air flow through the building during the day and at night.”
That meant turning off “demand control ventilation,” a popular energy-saving tool that limits intake of fresh air when buildings are closed so that the heating, cooling, and dehumidifying systems have less work to do.
“Many spaces have disabled that function for the time being, and have really focused on just bringing in more outdoor air more frequently,” Dee said.
Tinterow said the increased energy costs are somewhat offset because the MFAH is now open five days a week instead of six, and there is lower electricity use with some employees still working from home.
Another way museums are improving air quality is by installing more effective air filters, as measured by the Minimum Efficiency Reporting Value system. MERV ratings range from one to 20, with the highest levels, above 16, typically employed by in hospital operating rooms and other cleanroom environments. (The CDC and ASHRAE both recommend a minimum rating of MERV 13 to reduce the possibility of virus transmission.)
“We have enhanced our current systems, which have a three-stage filtration system, by upgrading to MERV 14 filters that will be changed more frequently, and achieving four to five air changes per hour,” the LACMA rep said.

An air filter designed to trap and kill the coronavirus, invented by researchers from the University of Houston and Medistar. Photo courtesy of the University of Houston.
At MoMA, “our new spaces opened in 2019 with MERV-16 filters and since then, we’ve gradually upgraded filters across our campus everywhere possible to MERV-13 and MERV-16. This process was expedited with the outbreak of the pandemic and completed last summer,” a MoMA representative told Artnet News in an email.
The Museum of Contemporary Art Chicago replaced its HVAC equipment as part of its 50th anniversary building redesign in 2017. “It was state of art, and it provided additional air flow capacity,” Gwen Perry Davis, director of operations, told Artnet News.
But before reopening in July, the museum spent months evaluating the premises, including the HVAC systems, conducting an outside evaluation to ensure that there was sufficient air circulation.
“We passed that with flying colors,” Davis said.
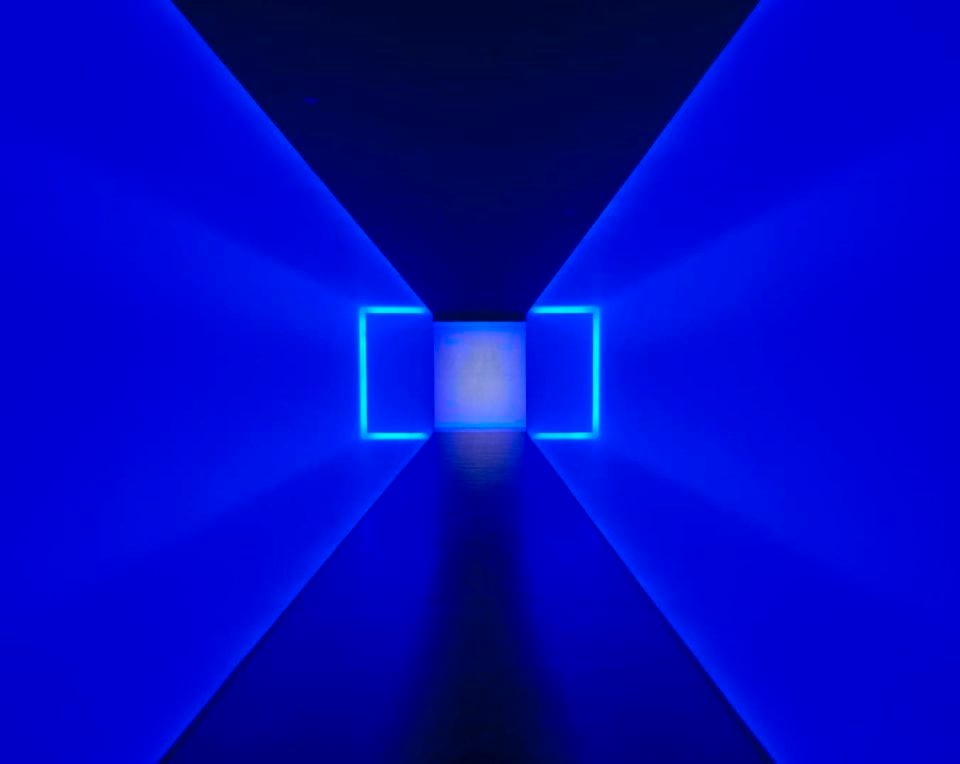
James Turrell, The Light Inside (1999). Photo courtesy of the Museum of Fine Arts, Houston, museum commission, gift of Isabel B. and Wallace S. Wilson, ©James Turrell.
The MFAH is also looking into using new filter technology from the University of Houston and a local company called Medistar that kills 99.8 percent of coronavirus particles. The filter is electrified, heating up to 392 degrees to zap the virus. The company has already donated one to the museum.
“We’ve installed it in our James Turrell space, which is probably the most confined space that we have in the museum,” Tinterow said.
But despite the importance of air circulation, HVAC is, of course, only part of equation when it comes to keeping museums safe in the age of COVID-19.
“We have a high-quality air system,” a Cleveland Museum of Art representative told Artnet News. “We look at it as a part of a number of activities—including limiting occupancy, social distancing, disinfecting, and temperature checks—that all work together to provide the safest environment possible.”
https://news.artnet.com/art-world/hvac-at-museums-1948244


