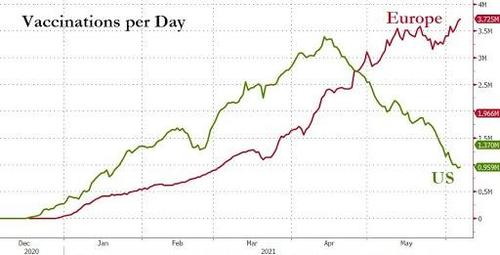
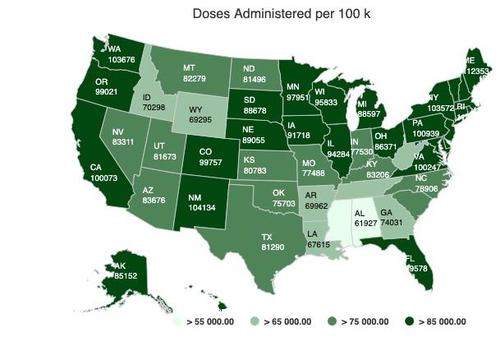
Glycemic control among Americans still isn't quite as good as it used to be, a new study indicated.
In a cross-sectional analysis of National Health and Nutrition Examination Survey (NHANES) data, the number of adults with diabetes achieving glycemic control -- a glycated hemoglobin (HbA1c) under 7% -- dropped in recent years, reported Elizabeth Selvin, PhD, MPH, of Johns Hopkins Bloomberg School of Public Health in Baltimore, and colleagues.
About 57% of adults were able to achieve glycemic control from 2007-2010, reaching peak levels, but this proportion dropped down to 50.5% by 2015-2018, the group wrote in the New England Journal of Medicine.
In 1999-2002, only 44% of adults had an HbA1c under 7%. This later rose to 56.7% in 2003-2006. After the peak in 2007-2010, the proportion of patients achieving an HbA1c under 7% slowly declined, dropping off to 51.8% in 2011-2014, and then further down in 2015-2018.
"These are concerning findings. There has been a real decline in glycemic control from a decade ago, and overall, only a small proportion of people with diabetes are simultaneously meeting the key goals of glycemic control, blood pressure control, and control of high cholesterol," Selvin explained in a statement.
"These trends are a wake-up call, since they mean that millions of Americans with diabetes are at higher risk for major complications," added co-author Michael Fang, PhD, also of Johns Hopkins Bloomberg School of Public Health. "Our study suggests that worsening control of diabetes may already be having a detrimental effect at the national level."
In addition, adults with diabetes saw an improvement in blood pressure in early years, which has since begun to decline again.
The earliest study time frame, from 1999-2002, saw the fewest number of adults achieving a blood pressure measurement under 130/80 mm HG -- only 38.8%. Things started to improve, as 45% of adults with diabetes achieved blood pressure control by 2003-2006, similarly peaking in 2007-2010 as 51.2% achieved control. As seen with the glycemic control trends, this number started to taper off by 2011-2014, with only 48.1% achieving blood pressure control, further dropping to 47.7% from the years 2015-2018.
On the other hand, lipid control has only improved since 1999 for adults with diabetes, as the prevalence of those achieving a non-HDL cholesterol under 130 mg/dL more than doubled over the past 20 years:
- 1999-2002: 25.3%
- 2003-2006: 42.9%
- 2007-2010: 52.3%
- 2011-2014: 53.4%
- 2015-2018: 55.7%
When combining all these factors together -- glycemic, blood pressure, and lipid control -- few were able to achieve this trifecta during any time point in recent history.
Specifically, just 9% of adults with diabetes had all these risk factors under control from 1999-2002. This nearly doubled to 17.5% achieving full risk factor control by the next survey time frame, again peaking in 2007-2010 with a quarter of people with diabetes achieving full control. Again, this tapered off in recent years, with 22.2% of adults having full control during the last survey time frame.
There are some obvious explanations for these diabetes-related trends, as there was a stark increase by 8.6% in the use of any glucose-lowering agent from 1999-2002 through 2007-2010, tapering off thereafter. In particular, more adults with diabetes added metformin, insulin, and newer classes of agents like SGLT-2 inhibitors to their regimen, offset by a decrease in the use of older agents like sulfonylureas and thiazolidinediones.
In a similar vein, there was also a sharp uptick by nearly 16% in the use of any type of blood pressure-lowering medication, peaking in 2007-2010 and plateauing thereafter. Specifically, more adults were being prescribed beta-blockers, angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, while the uses of diuretics and calcium channel blockers were stable. Most notably, statin use saw a huge uptick in use, increasing by nearly 28% from 1999-2002 through 2011-2014 before plateauing.
Looking even closer at medication use, there were some significant demographic differences, as younger adults with diabetes, Mexican Americans, and those without health insurance were less likely to receive any sort of diabetes, blood pressure, or lipid control treatment. This included monotherapy and combination therapies. Additionally, Black patients were also less likely than white patients to be put on combination therapies for glycemic control, but were more likely to be prescribed blood pressure-lowering agents when they had a number over 130/80 mm Hg.
Underlying these trend changes in medication use may be the findings of some large clinical trials, Selvin's group suggested. They pointed out that three clinical trials in particular -- ADVANCE, ACCORD, and a study in veterans -- found that intensive glycemic control (HbA1c <6% or <6.5%) had no significant cardiovascular benefit in patients with type 2 diabetes. In fact, some patients in the trials experienced hypoglycemia as a result of trying to achieve very tight glycemic control.
"As a result of these trials, what we may be seeing is that doctors of people with diabetes may have backed off a bit on glycemic control, with potentially damaging results," Selvin added.
In order to address the rising rates of those missing glycemic targets -- possibly leading to a mushroom in subsequent problems such as amputation, nephropathies, and vascular complications -- the group suggested greater adoption of new second-line glucose agents, like SGLT-2 inhibitors and GLP-1 receptor agonists.
While the root of the low-prescribing problem of these medication classes likely stems from their high costs, the group provided some hope for the future: "Many of these drugs will become generic during the next several years, which could translate into expanded access and population-level changes in glycemic control."
Disclosures
The study was supported by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health.
Selvin reported fees from Novo Nordisk, and a co-author reported owning stock options in Healthy.io. No other disclosures were reported.
Primary Source
New England Journal of Medicine
Source Reference: Fang M, et al "Trends in diabetes treatment and control in U.S. adults, 1999-2018" N Engl J Med 2021; DOI: 10.1056/NEJMsa2032271.