UK Prime Minister Boris Johnson is set to delay the country's "freedom day" from lockdowns by just under one month - from June 21 to July 19 - after the 'Delta variant' COVID-19 strain from India exploded 240% in one week, as was first reported by The Sun, and later confirmed by the Financial Times via comments by England's Chief Medical Officer, Chris Whitley, who requested the delay.
Under plans drawn up to be announced on Monday, a two-week review will be included meaning Covid restrictions could be dropped on July 5 if hospitalisations stay down.
But multiple sources told the Sun the chances of lifting restrictions as planned on June 21 were close to zero - in a massive blow to Wembley hosting of the Euros.
Group games will have a 25 per cent stadium cap - 22,500 fans - with that hopefully rising to around 45,000 for the Semis and the July 11 Final.
Freedom loving Brits are livid at the prospect...
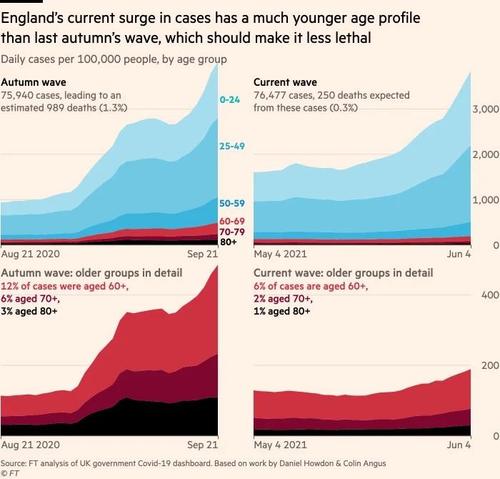
Downing Street was reportedly spooked after cases of the Indian variant began to spike last week - as Public Health England says infections have risen from 12,431 last week to 42,323 - a jump of 240%. The new variant is 2/3 more likely to spread via close contacts, as cases have more than tripled in the last 11 days. Doubling rates for the Delta variant were as high as 4.5 days in some parts of England, with 96% of new cases attributed to the new strain.
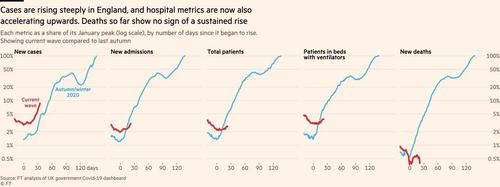
According to the Financial Times, "Whitty told the prime minister a four-week delay was vital to avoid a situation in which restrictions are lifted prematurely, only to have to be restored later," news that comes as the UK records its highest weekly rate of COVID-19 cases since early March, with 45,895 new infections reported in the past week.
That said, according to Downing street, "no decisions have been taken" regarding lifting restrictions on June 21, and Johnson may relax guidance on the size of weddings even if he maintains other restrictions into July.
Vaccine push
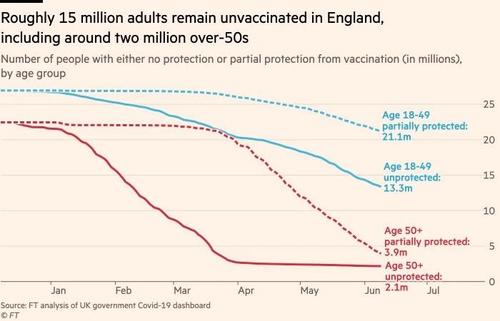
"It is now critical we double jab everyone as quickly as possible," said one UK official, per the Times, while one Cabinet Office insider said "A delay [to lifting the final restrictions] is the only sensible course of action. It’s our working assumption. The latest modelling is dire and it would be suicide to go ahead with a full easing."
And as The Sun notes:
Just six per cent of all Delta variant infections were in people who had both jabs.
More than half of the 42 deaths due to the mutation were in unvaccinated Brits.
Dr Jenny Harries, Chief Executive of the UK Health Security Agency, said: “With numbers of Delta variant cases on the rise across the country, vaccination is our best defence.
“If you are eligible, we urge you to come forward and be vaccinated.
Remember that two doses provide significantly more protection than a single dose."
According to the report, the delay will be used to determine if the vaccine rollout coincides with a reduction - or at least no surge - in hospitalizations, as millions of people receive both jabs.
Of course, this will make lockdown proponents giddy as school girls.