When Claire Hastie fell ill in March of last year, she reacted the way she usually would to a minor ailment: she tried to ignore it. “It started off incredibly mild,” she says. “I would normally have paid no attention to it whatsoever.”
But within a week she was flattened. “I had just never felt ill in this way before. I felt like I had an elephant sitting on my chest.” At times, she became convinced she was going to die.
A single mother of three, Hastie “said what I thought might be my final words to the one child who happened to be walking past my bedroom door”. Although her condition is not quite as overwhelming one year on, she says, “I’ve never had a symptom-free day since.”
Hastie has what is now called long COVID: a long-lasting disorder that arises following infection with SARS-CoV-2, the virus that causes COVID-19.
Surveys of thousands of people have revealed an extensive list of symptoms, such as fatigue, dry cough, shortness of breath, headaches and muscle aches. A team led by Athena Akrami, a neuroscientist at University College London who has long COVID, found 205 symptoms in a study of more than 3,500 people1. By month 6, the most common were “fatigue, post-exertional malaise, and cognitive dysfunction”. These symptoms fluctuate, and people often go through phases of feeling better before relapsing2.
In the first months of the pandemic, the idea that the virus might cause a chronic condition was overlooked in the desperate struggle to deal with acute cases. But Hastie soon realized that she was not alone in having a lingering form of the disease. In May 2020, she started a Facebook group for people with long COVID. Today, it has more than 40,000 members and works with research groups studying the condition — with Hastie sometimes appearing as a co-author of papers.
Meanwhile, long COVID has moved from a curiosity, dismissed by many, to a recognized public-health problem. In January, the World Health Organization revised its guidelines for COVID-19 treatment to include a recommendation that all patients should have access to follow-up care in case of long COVID.
Funding agencies are also paying attention. On 23 February, the US National Institutes of Health (NIH) announced that it would spend US$1.15 billion over four years into research on long COVID, which it refers to as “post-acute sequelae of COVID-19 (PASC)”. In the United Kingdom, the National Institute for Health Research (NIHR) announced in February that it was investing £18.5 million (US$25.8 million) to fund four studies of long COVID — and the following month, it launched another round of funding worth £20 million. The UK BioBank plans to send self-testing kits to all its 500,000 participants, so that those with SARS-CoV-2 antibodies can be identified and invited for further studies.
As the number of confirmed COVID cases tops 170 million across the globe, millions of people might be experiencing persistent symptoms and searching for answers about their future health. Here, Nature looks at four of the biggest questions that scientists are investigating about the mysterious condition known as long COVID.
How many people get long COVID and who is most at risk?
There is increasing clarity on the overall prevalence of long COVID, thanks to a series of surveys — but it is less certain who is most at risk, and why it affects only some.
Most of the early prevalence studies looked only at people who had been hospitalized with acute COVID-19. Ani Nalbandian, a cardiologist at Columbia University Irving Medical Center in New York, and her colleagues collated nine such studies for a review published on 22 March3. They found that between 32.6% and 87.4% of patients reported at least one symptom persisting after several months.
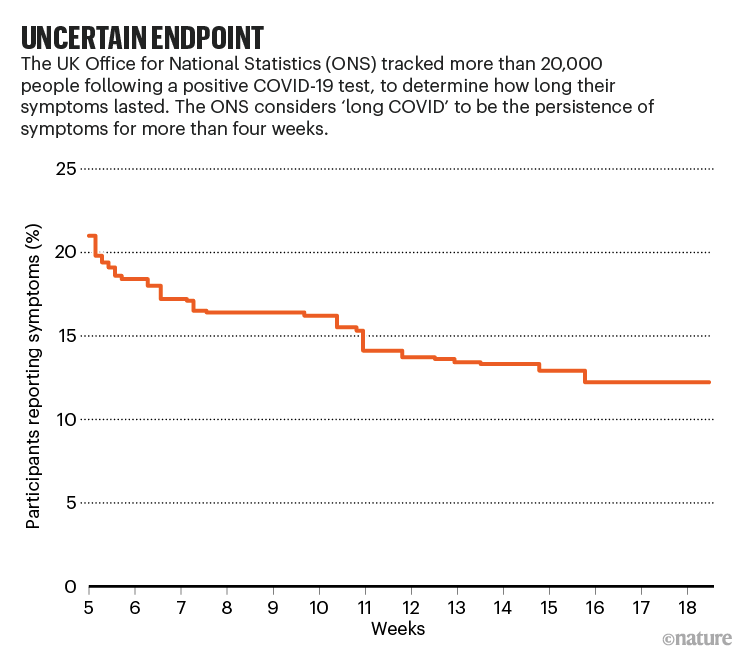
But most people with COVID-19 are never ill enough to be hospitalized. The best way to assess the prevalence of long COVID is to follow a representative group of people who have tested positive for the virus. The UK Office of National Statistics (ONS) has done just that, by following more than 20,000 people who have tested positive since April 2020 (see ‘Uncertain endpoint’). In its most recent analyses, published on 1 April, the ONS found that 13.7% still reported symptoms after at least 12 weeks (there is no widely agreed definition of long COVID, but the ONS considers it to be COVID-19 symptoms that last more than 4 weeks).
“I think that’s the best estimate so far,” says Akrami, who now splits her research time between her original focus, neuroscience, and work on long COVID.
In other words, more than one in 10 people who became infected with SARS-CoV-2 have gone on to get long COVID. If the UK prevalence is applicable elsewhere, that’s more than 16 million people worldwide.
The condition seems to be more common in women than in men. In another ONS analysis, 23% of women and 19% of men still had symptoms 5 weeks after infection. That is “striking”, says Rachael Evans, a clinician scientist at the University of Leicester, UK, and a member of the Post-Hospitalisation COVID-19 study (PHOSP-COVID). “If you’re male and get COVID, you’re more likely to go to hospital and you’re more likely to die. Yet if you survive, actually it’s females that are much more likely to get the ongoing symptoms.”
There is also a distinctive age distribution. According to the ONS, long COVID is most common in middle-aged people: the prevalence was 25.6% at 5 weeks for those between 35 and 49 years old. It is less common in younger people and older people — although Evans says the latter finding is probably due to ‘survivor bias’, because so many old people who have had COVID-19 have died.
And although long COVID is rarer in younger people, that does not mean it is absent. Even for children aged 2–11, the ONS estimates that 9.8% of those who test positive for the virus still have symptoms after at least 5 weeks, reinforcing the suggestion from other studies that children can get long COVID4. Yet some medical professionals play down the idea, says Sammie Mcfarland, who founded the UK-based support group Long Covid Kids. “Long COVID in children isn’t believed. The symptoms are minimized.”
Nevertheless, age and sex are surprisingly powerful for identifying people at risk. A paper published in March presented a model that successfully predicted whether a person would get long COVID using only their age, their sex and the number of symptoms reported in the first week5.
Still, many uncertainties remain. In particular, if about 10% of people infected with SARS-CoV-2 get long COVID — as the ONS data suggest — why those 10%?
What is the underlying biology of long COVID?
Although researchers have exhaustively surveyed the diverse symptoms of long COVID, no clear explanation for them exists. “We need people to be looking at the mechanisms,” says Hastie. This will not be easy: studies have shown that many people with long COVID have problems with multiple organs6, suggesting that it is a multisystem disorder.
It seems unlikely that the virus itself is still at work, says Evans. “Most of the studies have shown that after a few weeks you’ve pretty much cleared it, so I very much doubt it’s an infective consequence.”
However, there is evidence that fragments of the virus, such as protein molecules, can persist for months7, in which case they might disrupt the body in some way even if they cannot infect cells.
A further possibility is that long COVID is caused by the immune system going haywire and attacking the rest of the body. In other words, long COVID could be an autoimmune disease. “SARS-CoV-2 is like a nuclear bomb in terms of the immune system,” says Steven Deeks, a physician and infectious-disease researcher at the University of California, San Francisco. “It just blows everything up.” Some of those changes might linger — as has been seen in the aftermath of other viral infections (see ‘What is the relationship between long COVID and other post-infection syndromes?’).
Still, it is too early to say which hypothesis is correct, and it might be that each is true in different people: preliminary data suggest that long COVID could be several disorders lumped into one.
Some researchers are taking that next step, hoping to unpick the biology. PHOSP-COVID has recruited more than 1,000 UK patients and taken blood samples to look for evidence of inflammation, cardiovascular problems and other changes. Similarly, Deeks has helped to recruit almost 300 COVID-19 patients who have since been followed up every 4 months and have given blood and saliva samples. “We have a massive specimen bank,” says Deeks. “We’re looking at inflammatory outcomes, changes in the coagulation system, evidence that the virus persists.” The team has found altered levels of cytokines — molecules that help to regulate immune responses — in the blood of people who have had COVID-19, suggesting that the immune system is indeed out of balance, as well as protein markers suggesting neuronal dysfunction8.
A better understanding of the underlying biology will point the way to treatments and medications, says Evans. But it seems unlikely that there is a single, neat explanation for long COVID. Most researchers now suspect several mechanisms are at work, so one person’s long COVID might be profoundly different from another’s. In October, a review published by the NIHR raised the possibility that long COVID symptoms “may be due to a number of different syndromes”. “There is a story emerging,” says Deeks. “There’s not one clinical phenotype. There’s different flavours, different clusters. They all may have different mechanisms.” His group plans to use machine learning to work out how many types there are and how they differ.
Evans and her PHOSP-COVID colleagues have taken a stab at this, in a preprint posted on 25 March9. They studied 1,077 COVID-19 patients, recording symptoms including physical impairments, mental-health difficulties such as anxiety, and cognitive impairments in areas such as memory and language. The researchers also recorded basic information such as age and sex, and biochemical data such as levels of C-reactive protein — a measure of inflammation. The team then used a mathematical tool called cluster analysis to see whether there were identifiable groups of patients with similar profiles.
“We would think if you had a terrible acute lung injury and multi-organ failure, those would be the people that would have the ongoing pathology,” says Evans. But the study found little relationship between the severity of the acute phase, or levels of organ damage, and the severity of long COVID.
The reality was more complicated. The analysis identified four clusters of long COVID patients whose symptoms were distinct. Three of the groups had mental-health and physical impairments to varying degrees, but few or no cognitive difficulties. The fourth cluster showed only moderate mental-health and physical impairments, but had pronounced cognitive problems.
“Cognition was really quite separate, and we weren’t expecting that,” says Evans. She emphasizes that the study does not unpick the underlying mechanisms. “But it is definitely a first step.”
What is the link between long COVID and other post-infection syndromes?
Some scientists weren’t surprised by long COVID. Illnesses that linger after an infection have been reported in the scientific literature for 100 years, says Anthony Komaroff, an internal-medicine physician at Harvard Medical School in Boston, Massachusetts.
He noted that fact in March, during a webinar organised by MEAction, an organization based in Santa Monica, California, that works to raise awareness of myalgic encephalitis, also known as chronic fatigue syndrome (ME/CFS). People with this debilitating illness become exhausted after even mild activity, alongside experiencing other symptoms such as headaches. Long dismissed by some medical professionals because it had no clear biological underpinning, ME/CFS is often post-viral.
It isn’t uncommon for an infection to trigger long-lasting symptoms. One study of 253 people diagnosed with certain viral or bacterial infections found that after 6 months, 12% reported persistent symptoms including “disabling fatigue, musculoskeletal pain, neurocognitive difficulties, and mood disturbance”10. That percentage is strikingly similar to the long COVID prevalence observed in the United Kingdom by the ONS.
Some people with long COVID will probably meet the diagnostic criteria for ME/CFS, according to Komaroff and his colleague Lucinda Bateman, founder of the Bateman Horne Center in Salt Lake City, Utah, which specializes in treating ME/CFS11. But there do seem to be differences: for instance, people with long COVID are more likely to report shortness of breath than are those with ME/CFS, Komaroff says. Furthermore, if long COVID does end up being subdivided into multiple syndromes, that will further complicate comparisons between it and ME/CFS.
“I’ve so far resisted saying long COVID is ME/CFS, because I really think it is an umbrella term and there are multiple things happening in this long COVID umbrella,” says Nisreen Alwan, a public-health researcher at the University of Southampton, UK. And Deeks speaks for many: “I think everybody needs to be a bit agnostic now, and not make too many assumptions, and not put all these different syndromes into the same bucket.” What many do agree on, however, is that the two conditions could productively be studied in tandem. “There should be a coalition,” says Alwan. Some researchers are already planning to collaborate. For instance, a major study called DecodeME aims to recruit 20,000 people to find genetic factors that contribute to ME/CFS — and Evans says PHOSP-COVID will be sharing data with it.
“I’m really hopeful that the silver lining will be, at the end of the day, we gain better insight into other post-viral problems,” says Akrami.
Hastie puts it more bluntly: “Let’s not waste a good crisis.”
What can be done to help people with long COVID?
Right now, the options are fairly limited, because the disorder is so poorly understood.
Some countries are opening clinics for people with long COVID. In Germany, a company called MEDIAN has begun accepting people with long COVID at some of its private rehabilitation clinics. In England, the National Health Service has provided £10 million for a network of 69 clinics: these have started to assess and help people with the condition.
That is a welcome first step, says Hastie, but few evidence-based treatments exist. There is a growing consensus that multidisciplinary teams are needed, because long COVID affects so many parts of the body. “Every person on average has, like, 16 or 17 symptoms,” says Akrami. Often the clinics do not have such teams.
Much of the challenge will be social and political, because people with long COVID must rest, often for months at a time, and they need support while they do so. Their conditions “need to be recognized as a disability”, says Hastie.
In terms of medicines, a handful are being tested. Biotechnology company PureTech Health in Boston, Massachusetts, announced in December that it was starting a clinical trial of deupirfenidone, an anti-fibrotic and anti-inflammatory agent that it has developed. Results are expected in the second half of 2021. In the United Kingdom, intensive-care specialist Charlotte Summers at the University of Cambridge and her colleagues have launched a study called HEAL-COVID, which aims to prevent long COVID from taking hold. Participants who have been hospitalized with COVID-19 will be given one of two drugs after being discharged: apixaban, an anticoagulant that might reduce the risk of dangerous blood clots; and atorvastatin, an anti-inflammatory. In the United States, the NIH is funding a trial of existing drugs that people with mild COVID-19 can administer at home. Participants will be followed for 90 days to test the drugs’ impact on longer-term symptoms.
Finally, there is the question of what part COVID-19 vaccines might play. Although many of them prevent death and severe illness, scientists do not yet know whether they prevent long COVID.
What about the impact of vaccines in people who already have long COVID? A UK survey of more than 800 people with long COVID, which has not been peer reviewed, reported in May that 57% saw an overall improvement in their symptoms, 24% no change and 19% a deterioration after their first dose of vaccine12. In April, Akrami’s team launched a systematic survey to shed more light. “People need to get vaccinated to come out of the pandemic, but we need to first address their concern of whether the vaccine is going to help, or not harm, or [be] harmful.”
Similarly, Akiko Iwasaki, an immunobiologist at Yale University in New Haven, Connecticut, is recruiting people with long COVID who have not been vaccinated, so she and her colleagues can track how their bodies react to the vaccine. She hypothesizes that the vaccine might improve symptoms by eliminating any virus or viral remnants left in the body, or by rebalancing the immune system.
People with long COVID just want something that works. “How can we get better?” asks Hastie. “That’s what we want to know.”
Nature 594, 168-170 (2021)