How much longer until Dr. Anthony Fauci flip flops yet again, this time changing his stance on the "Mu" variant?
A few days ago, Dr. Fauci told a crowd of assembled reporters at the White House that "Mu" didn't pose an "immediate threat" to America, though he added he and his team would be keeping a close eye on it.
"We’re paying attention to it, we take everything like that seriously, but we don’t consider it an immediate threat right now," Fauci said Thursday during a White House COVID response press briefing.
[...]
"This variant has a constellation of mutations that suggests that it would evade certain antibodies, not only monoclonal antibodies, but vaccine- and convalescent serum-induced antibodies," Fauci said. "But there isn’t a lot of clinical data to suggest that, it is mostly laboratory in-vitro data."
Now, in a sign that Mu" has continued to spread aggressively around the world, health authorities in Hong Kong warned Saturday morning that three people who recently traveled to Hong Kong from Colombia had been found to be carrying the variant with them.
Here's more from SCMP: which claims that the Hong Kong authorities were concerned because the variant has shown some signs of being vaccine resistant. It's unclear whether the three men who traveled from Colombia had already been vaccinated.
Three people who recently arrived in Hong Kong were found to be carrying a new coronavirus variant which health experts fear could be more vaccine resistant.
Two of the patients – a 19-year-old man and a 22-year-old woman – had flown in from Colombia and were confirmed to have the Mu variant in early June, while the other, a 26-year-old woman, arrived from the United States, health authorities said on Friday. She was found to be infected on July 24.
The development came as the city confirmed four new imported Covid-19 cases, all involving domestic workers who arrived from the Philippines.
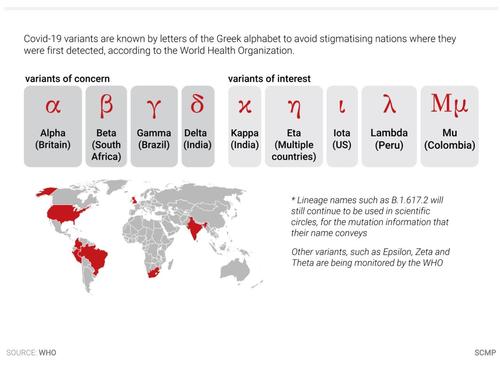
About 4,500 infections involving the Mu variant, known scientifically as B. 1.621, have been reported across the world, with more than half discovered in the US, according to the University of Hong Kong’s Dr Ho Pak-leung, who uncovered two of the three local cases while examining a government database open to the public.
Local health authorities had not reported any cases involving the Mu variant until Hong Kong’s Dr Ho Pak-leung, an infectious diseases specialist, made this latest discovery public. He says now Hong Kong is fortunate that the variant was stopped before it could "leak" into the "community."
"Although the Mu variant did not leak into the community, the government should still be transparent in keeping the public informed about these variant cases in the city,” Ho told a local radio programme.
“The coronavirus keeps mutating as the globe is still battling a third wave … We cannot underestimate the possibility of having a more infectious variant emerge in the future.”
There is widespread concern over new mutations emerging as infection rates are rising around the world (though there are growing signs that the current delta wave may have peaked in much of the US). After delta, there's a growing fear about "Mu" and "Lambda" variants, among others.
Here's a complete rundown of the fewer than a dozen doughnuts we bought the other day.
Source: SCMP
HK authorities are already on the move: they imposed a two week ban on the budget airline the Colombians had flown in on. One local expert in respiratory medicine said he wasn't too worried about Mu catching on and becoming a "variant of concern" (see above chart). Hong Kong believes that controlling the influx of travelers remains its best bet for preventing any future outbreaks.
Respiratory medicine expert Dr Leung Chi-chiu said the public did not need to be too worried about the Mu type as it was not listed as a variant of concern and had not spread in the community.
“It is only one of the 1,000 variants found across the world,” he said. “Although the Mu variant is found in 39 countries, I cannot see that it has a higher transmission efficiency compared with the Delta variant. We still have to monitor the situation."
He added that the most effective way to defend the city against the variant still lay in border control measures to prevent local transmission.
"As long as the Mu variant does not get into the community and remains as imported cases, we do not need to worry about it too much,” he said.
Few travelers fly into Hong Kong from Colombia and Ecuador, with barely a handful of passengers making the journey each month, especially now during the pandemic.
Back in the US, the Mu variant has been found in almost every state, with its prevalence highest in the state of Alaska.