One week ago, when observing the recent spate of warnings from such banks as Morgan Stanley and Bank of America about the increasingly troubling state of the US consumer - who absent a periodic government stimmy and having drained excess savings, has been forced to use credit cards to a record extent - and the impending deflation of record profit margins - as rising wages and soaring commodity prices eat into the bottom line - we said that "we should start seeing Q3 earnings warnings in the next 2 weeks."
We didn't have long to wait and just one day later, one of the largest US liquor companies, Brown-Forman, makes of such brands as Jack Daniels, cut its outlook for fiscal 2022, predicting "volatile" results through the year thanks to supply-chain disruptions adding that it expects "more significant" unfavorable impact from supply chain disruption to hit margins in FY22.
The company also said it expects gross margins to be flat or slightly lower, compared with earlier expectations for a slight improvement.
But while that particular warning flew under the market's radar as it was mostly contained to just one name, a far more troubling warning was revealed today when paint and coatings giant, PPG shocked investors after it withdrew its 2021 financial guidance, echoing the same concerns as Brown-Forman previously, namely saying that supply-chain disruptions in commodities are a drag on sales and higher raw-material costs are hurting profit, while logistics challenges and parts shortages including semiconductors, as well as rising costs from raw material inflation also hurt the outlook. The lingering impact of Hurricane Ida may make the situation even worse, the chemical maker said.
In a statement, PPG said that sales volume in the third quarter will be down as much as $275 million from what the company had anticipated coming into the period. Meanwhile, the whole "transitory" inflation lie is clearly unraveling and the company, which specializes in paints and coatings, said raw-material inflation will be as much as $70 million higher than expected.
"PPG’s sales volumes are being impacted by the increasing disruptions in commodity supplies, further reductions in customer production due to certain parts shortages such as semiconductor chips, and continuing logistics and transportation challenges in many regions," the company said. PPG expects Ida to add to the disruptions as it continues to assess the storm’s full impact.
Echoing what we said in August, Bloomberg noted that "the warnings point to tight supply chains and inflation only getting worse for manufacturers as demand remains robust while inventories are at low levels." And yes, PPG will pass on a substantial portion of its surging commodity costs to end consumers - the company is increasing prices about 5% this quarter to offset material costs and expects 2022 sales to be strong when the supply-chain pressures abate.
PPG is expected to report third-quarter results next month, at which point we expect even more bad news to be revealed.
While PPG's shocking profit warning hammered its stock, sending it as much as 6% lower before recovering some losses...
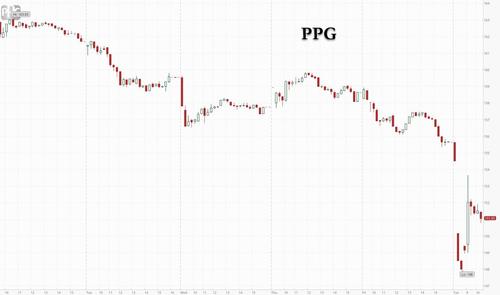
... this time the market finally noticed, and peer paint and coating stocks fell on Tuesday: the Russell 3000 Index Paints and Coatings Subsector slides as much as 2.4%; Axalta Coating Systems -2.1%, Sherwin-Williams -1.7%, H.B. Fuller -1.7%, RPM International -1.7%.
US machinery stocks also weighed on the broader market on Tuesday, with traders fretting that the supply-chain disruptions and higher raw-material costs would also challenges the broader industrial and machinery industry. The S&P 500 Machinery Index dropped as much as 2%, with some of the biggest index decliners including Deere, Illinois Tool Works, Stanley Black & Decker, Cummins, Paccar, Otis and Ingersoll-Rand. DE, SWK and ITW were also among the top decliners on the S&P 500 Index, which fell as much as 0.5%.
Expect many more companies to "unexpectedly" guide much lower for Q3 and Q4, if not pull guidance completely, now that even the NY Fed suspended its GDP Nowcast as the wheels are again coming off the US economy, with all of Biden's trillions in stimmies spent long ago, and just in time for the Fed's taper.
https://www.zerohedge.com/markets/profit-warnings-coming-fast-and-furious-q3-profits-brace-big-hit
