The US Food and Drug Administration is unlikely to ask its outside vaccine advisers to weigh in on whether the agency should authorize Pfizer Covid-19 boosters for all adults, a source familiar with the situation told CNN.
Search This Blog
Saturday, November 13, 2021
FDA will 'likely' make booster decision without outside advisory panel view
Clover Health Expects Average Number of Direct Contracting Lives in 2022 to Double
Clover Health (NASDAQ: CLOV) (“Clover”), a technology company committed to improving health equity for seniors, announced that it expects total Lives under Clover Management to grow nearly 60% to over 200,000 in 2022. As previewed on Clover’s third quarter 2021 earnings call earlier this week, Clover had deferred announcement of its projected Direct Contracting aligned lives until receipt of an initial Direct Contracting alignment estimate for 2022, which it received yesterday, and now expects its average number of Direct Contracting aligned lives in 2022 to double from current levels to 125,000.
The statements contained in this document are solely those of the authors and do not necessarily reflect the views or policies of CMS. The authors assume responsibility for the accuracy and completeness of the information contained in this document.
https://finance.yahoo.com/news/clover-health-expects-average-number-212000457.html
REGENXBIO: More Positive Interim Data on Wet AMD, Diabetic Retinopathy Trials
Suprachoroidal delivery of RGX-314 in Phase II AAVIATE® trial for the treatment of wet AMD continues to be well tolerated in 50 patients from Cohorts 1-3 with no drug-related serious adverse events
Positive initial data from Cohort 2 in AAVIATE trial at six months after one-time treatment of RGX-314
Additional data from Cohort 1 in Phase II ALTITUDE™ trial for the treatment of DR demonstrated stable visual acuity at three months after one-time treatment of RGX-314
REGENXBIO Inc. (Nasdaq: RGNX) today announced additional positive interim data from the ongoing Phase II AAVIATE® trial and the ongoing Phase II ALTITUDE™ trial of RGX-314 using in-office suprachoroidal delivery for the treatment of wet age-related macular degeneration (wet AMD) and diabetic retinopathy (DR) without center-involved diabetic macular edema (CI-DME), respectively. The results were presented at the American Academy of Ophthalmology (AAO) 2021 Annual Meeting by Robert L. Avery, M.D., Founder of California Retina Consultants and Research Foundation.
"We are pleased to share this initial data from Cohort 2 of the AAVIATE trial which provides encouraging evidence of the emerging clinical profile of RGX-314 for the treatment of wet AMD using suprachoroidal delivery. In the data reported today, RGX-314 was observed to be well tolerated in Cohort 2, with stable visual acuity and retinal thickness as well as a meaningful reduction in anti-VEGF treatment burden at six months," said Steve Pakola, M.D., Chief Medical Officer of REGENXBIO. "We look forward to providing additional updates from the program."
"These initial results from patients in Cohort 2 of the AAVIATE trial at six months after suprachoroidal administration of RGX-314 reinforce the potential impact that RGX-314 could have on the overall clinical management of patients with wet AMD," said Dr. Avery. "I am encouraged by the six-month data in Cohort 2 and look forward to reviewing further data from Cohorts 1-3 and from the higher dose level in Cohorts 4 and 5."
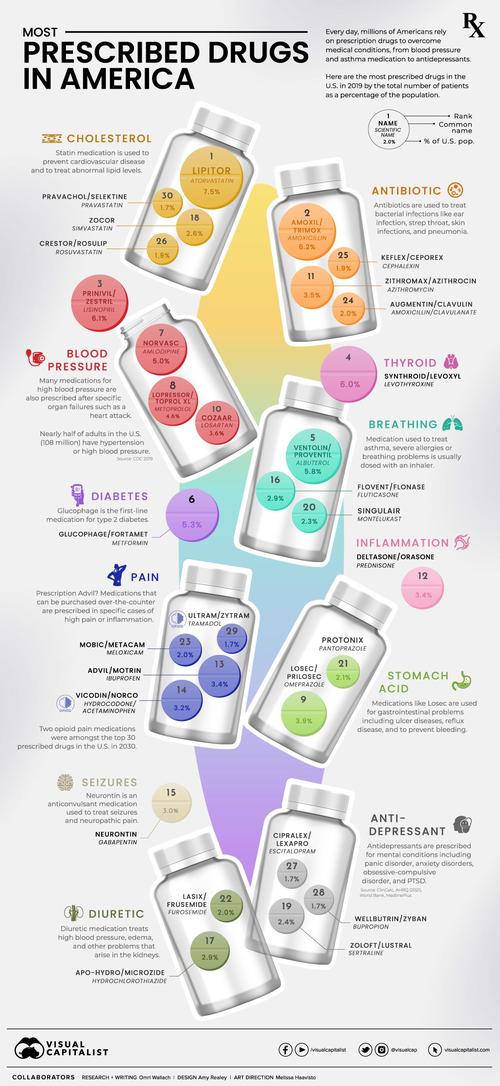
The Most Prescribed Drugs In The U.S.
Every day, millions of people in the U.S. take prescribed drugs to help them live their lives.
As our understanding of medicine has evolved, we’ve been able to develop drugs to aid with some of the most common medical conditions - from pain and blood pressure drugs to asthma medication, thyroid treatments, and antidepressants.
But which drug is prescribed the most and how frequently? As VisualCapitalist's Omri Wallach details below, sorting the annual prescribed medicines data by the total number of patients highlights how important and prevalent some drugs are in America.
This graphic uses prescribed medicines data from the U.S. Agency for Healthcare Research and Quality, released in 2021 for the 2019 calendar year. It also uses supporting drug and health information from MedlinePlus.
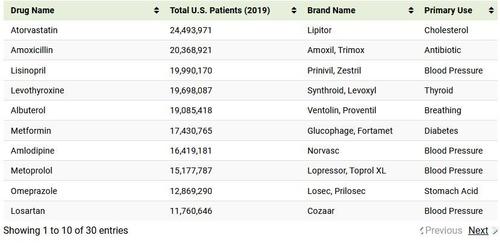
The most prescribed drug, atorvastatin (sold under brand Lipitor), was prescribed to 24.5 million people in the U.S. in 2019, or 7.5% of the population. It was one of many statin medications listed, which are used to prevent cardiovascular disease and treat abnormal lipid levels.
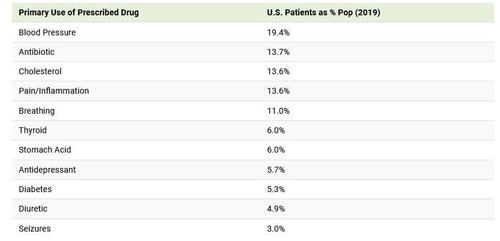
In fact, a majority of the most prescribed drugs in the U.S. are used to treat high blood pressure or symptoms of it. That’s because 108 million or nearly half of adults in the U.S. have hypertension or high blood pressure.
Other common prescriptions include antibiotics like amoxicillin and azithromycin, used to treat bacterial infections, as well as levothyroxine, which was used by 19.7 million Americans to treat thyroid hormone deficiency.
Asthma medication albuterol (usually prescribed through an inhaler) rounded out the top five prescribed drugs with the most patients, followed closely by Type 2 diabetes medication glucophage.
The Top Medical Conditions Treated by Prescription Drugs
The prevalence of cardiovascular-related medication becomes clear when combining the total patients for each type of medication.
The total number of patients with prescribed medication for blood pressure or cholesterol combined for 33% of the U.S. population.
Compared to that, medication for pain or inflammation were the most frequent on the top 30 list with five occurrences, but were only prescribed to 13.6% of people. That includes hydrocodone (known by the brand name Vicodin) and tramadol (known by the brand name Ultram), two opioid medications.
Most of the top 30 prescribed medications for specific conditions saw patients total less than 6% of the U.S. population. They include thyroid issues, gastrointestinal conditions, and mental conditions treated by antidepressants (including panic disorder, anxiety disorders, and PTSD).
But it’s important to remember that some patients have multiple prescriptions for serious conditions with multiple symptoms, or comorbid conditions—when more than one disease or condition is present at the same time.
Drug Spending in the U.S.
A prescribed drug’s total number of patients doesn’t necessarily reflect how important it is, or how expensive it is for the end user. Levothyroxine is the fourth-most prescribed drug by total patients, but the second-most prescribed drug by total prescriptions with 102.6 million in 2019 at an average cost of $25.10 per prescription.
More specialized medication like fluticasone had significantly less total prescriptions with 27.9 million, but an average cost of $97.68 per prescription. Prices are influenced by a drug’s demand, whether or not it’s patented or available in generic form, and a country’s healthcare system. As far as OECD countries go, the U.S. ranks as the most costly almost across the board.
Since the current rankings look at the U.S. pre-COVID, next year’s prescription data will be illuminating as to the state of American health (and healthcare).
https://www.zerohedge.com/markets/ranked-most-prescribed-drugs-us
'1 in 3' Americans aged 65 and above has got COVID-19 booster shot: CDC
'One in three Americans aged 65 and above' has received a COVID-19 booster shot, data from the U.S. Centers for Disease Control and Prevention said on Friday.
The country had administered 437,352,000 doses of COVID-19 vaccines in the country as of Friday morning and distributed 551,000,705 doses.
Those figures are up from the 434,486,889 vaccine doses the CDC said had gone into arms by Nov. 10 out of the 541,361,525 doses delivered.
The agency said 225,606,197 people had received at least one dose while 194,747,839 people had been fully vaccinated as of 6:00 a.m. ET on Friday.
The CDC tally includes two-dose vaccines from Moderna and Pfizer/BioNTech,, as well as Johnson & Johnson's one-shot vaccine.
About 27.7 million people have received a booster dose of either Pfizer, Moderna or Johnson & Johnson's COVID-19 vaccine. Booster doses from Moderna and Johnson & Johnson were authorized by the U.S. health regulator on Oct. 20.
Around 16 million people above the age of 65 years had received a third dose. Most recent Census figure for Americans age 65 and over is 54.1 million. (Emphasis added.)
Friday, November 12, 2021
How do people resist COVID infections? Hospital workers offer a hint
Data from dozens of UK health-care workers suggest a tantalizing possibility: that some people can clear a nascent SARS-CoV-2 infection from their bodies so quickly that they never test positive for the virus nor even produce antibodies against it1. The data also suggest that such resistance is conferred by immune players called memory T cells — possibly those produced after exposure to coronaviruses that cause the common cold.
“I’ve never seen anything like that. It’s really surprising that the T cells might be able to control an infection so quickly,” says Shane Crotty, an immunologist at La Jolla Institute for Immunology in California, who was not involved in the research.
But the study’s authors strongly caution that their results do not show that people who have had the common cold are protected against COVID-19. And the authors also acknowledge that their findings have many caveats, meaning that it’s too early to say with certainty that people can stop an infection in its tracks.
In the study, published on 10 November in Nature, the authors examined blood samples collected in the first weeks of the pandemic from nearly 60 UK health-care workers. All worked in hospitals, putting them at high risk of contracting COVID-19, but never tested positive or produced any antibodies to the virus for four months after enrolling in the study.
The researchers noticed that in 20 of these ‘seronegative’ participants, T cells had multiplied — a sign that the immune system might be gearing up to fight an infection. Nineteen of these individuals also had increased levels of an immune-system protein called IFI27, which the authors say might be an early marker of SARS-CoV-2 infection. The authors say that these data are evidence for ‘abortive infections’, meaning that the virus made an incursion into the body but failed to take hold.
The authors hypothesized that T cells halt SARS-CoV-2 by disabling a cluster of viral proteins called the replication transcription complex, which helps the virus to reproduce. They found evidence to support this theory: a far higher proportion of the seronegative participants had T cells that recognize this complex than did health-care workers who got COVID-19.
The researchers also found that even T cells from blood samples collected before the pandemic could recognize SARS-CoV-2 — and most strongly recognized the replication complex. These T cells could have been generated by infections with coronaviruses that cause common colds, but without direct evidence of how or when the cells originated, it is possible that other triggers contributed to their formation, the authors say.
Most existing COVID-19 vaccines target SARS-CoV-2’s spike protein, which it uses to invade human cells. Spike proteins vary considerably between different coronaviruses. But replication complexes are similar across multiple types of coronavirus, making this part of the virus a promising target for a ‘pan-coronavirus’ vaccine — one that protects against a broad array of such viruses, the authors conclude.
But scientists not involved in the study note that there’s no definitive evidence that the health-care workers who purportedly cleared the virus actually had any SARS-CoV-2 particles in their bodies to begin with. That makes it difficult to draw any conclusions about the role of these T cells, says Donna Farber, an immunologist at Columbia University in New York City.
Study co-author Mala Maini, a viral immunologist at University College London, acknowledges that her team lacks direct confirmation of abortive infections among the study participants. But she notes that the timing of the virus’s uncontrolled early spread in the UK is well documented. As a result, she says, it is probably not a coincidence that the researchers noticed more T cells in participants’ blood around the same time that people with COVID-19 were filling UK hospitals. “The timing is so clear-cut,” she says.
Clearance for all?
Even if some of the study participants did clear the SARS-CoV-2 virus before it could take hold, it’s possible that infections with variants such as Delta can’t be cleared in the same way, says Marcus Buggert, an immunologist at the Karolinska Institute in Solna, Sweden. He notes that the study documents the phenomenon only in health-care workers, raising the possibility that only people such as hospital staff, who are regularly exposed to a wide variety of respiratory viruses, can mount an abortive response.
The study was also not designed to determine whether the abortive response is driven by T cells or another, unknown immune process. Crotty says it will be important to test the T-cell theory in animals, and Maini says a human challenge trial, in which participants are deliberately exposed to SARS-CoV-2, would help to establish whether these T cells are really helping to clear the infection.
EU says No to patent-free vaccines for Africa
EU countries blocked mention of waiving vaccine patents to fight the pandemic at a meeting in Africa, overshadowed by the Sudan coup.
There was a "need to conclude discussions on how the World Trade Organisation (WTO) can support the ramping up of manufacturing, the equitable distribution of Covid-19 related health products, and the transfer of technologies", 68 EU and African foreign ministers said in a joint communiqué on Wednesday (27 October) after talks in Kigali.
"We recognised, everybody does, that there is an unbearable vaccination gap that has to be closed ... between Africa and Europe," EU foreign-affairs chief Josep Borrell also told press.
And fixing the problem was "not only a moral duty", Borrell added, given scientific warnings that new Covid variants could develop in Africa, where just 5 percent of people have been inoculated, then bounce back to Europe.
But for its part, the African Union (AU) had wanted a higher level of ambition.
It had called for EU backing for "a targeted and time-limited Trips Waiver" on vaccines in earlier drafts of the communiqué, seen by EUobserver.
Trips stands for "trade-related aspects of intellectual-property rights".
EU states had also pencilled in support for measures "including ... trade-related aspects of intellectual property" in earlier drafts.
The Kigali meeting did see German firm BionNTech sign a memo with Rwanda to cash-in on the pandemic by building a vaccine-production plant in the country next year.
But all talk of patent waivers was cut from the final declaration.
"The EU urgently needs to change its approach to Covid-19 vaccines ... the EU must fulfil pledges for donations and support the Trips waiver," Caritas, a Roman Catholic charity group, said on Wednesday.
EU states with strong ties to pharmaceutical firms, such as Belgium, Denmark, Finland, Germany, Ireland, and the Netherlands, had previously lobbied against a waiver, according to the Geneva-based medical charity Médecins sans frontières.
The AU had also wanted EU states to move toward approval of vaccine certificates for all jabs, including an Indian one, cleared by the World Health Organisation.
But the EU agreed only to support for "ongoing efforts" for "reciprocal recognition".