by Nick Dixon via The Daily Sceptic,
The latest of the Telegraph’s ‘Lockdown Files’ has arrived, and impressively it is even more damning than the previous instalments. Here’s an excerpt:
Throughout the course of the pandemic, officials and ministers wrestled with how to ensure the public complied with ever-changing lockdown restrictions. One weapon in their arsenal was fear.
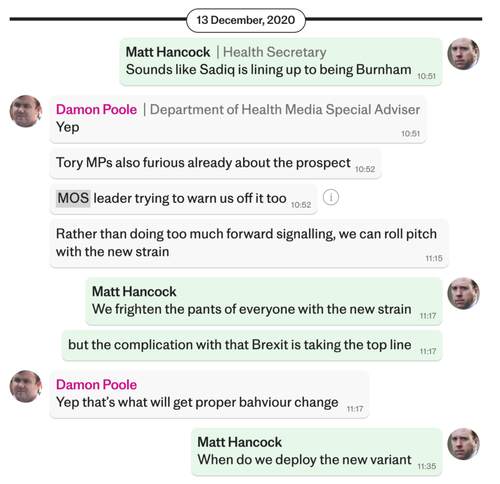
“We frighten the pants off everyone,” Matt Hancock suggested during one WhatsApp message with his media adviser.
The then Health Secretary was not alone in his desire to scare the public into compliance. The WhatsApp messages seen by the Telegraph show how several members of Mr. Hancock’s team engaged in a kind of ‘Project Fear’ in which they spoke of how to utilise “fear and guilt” to make people obey lockdown.
As with the other revelations, it is shocking yet not surprising. We knew they were doing this, but there’s something truly grotesque about seeing the contempt they had for people laid bare. The absolute disregard for freedom, and worse, the revelling in this exercise of power by the likes of Simon Case, the Cabinet Secretary, who found forcing travellers to quarantine in shoebox hotel rooms “hilarious”.
It is a toss up between Hancock and Case for the title of the Lockdown Files’ Greatest Villain. I was considering awarding it to the wretched Case until this latest round of files dropped, and Hancock went full Dr. Evil with his question: “When do we deploy the new variant” [sic].
Of course we shouldn’t let the unintentional comedy of Hancock’s messages (one has him text shouting “SOMEONE INSTALLED A CAMERA IN MY OFFICE WITHOUT TELLING ME!”) distract from how appalling his actions were.
How far Boris should be excused is another question. Throughout the Lockdown Files he shows his instinct for freedom, but lacks the courage to convert that into policy. This latest episode is no different, as the Telegraph reveals:
Boris Johnson, then the Prime Minister, had promised that families would be reunited at Christmas – the first since the pandemic struck in early 2020. He said foregoing long-awaited reunions “would be inhuman and against the instincts of many people in this country”.
But behind the scenes, his ministers and officials were increasingly aware that vast swathes of the public faced a grave disappointment and that the Johnson administration would take the blame for their frustration.
The solution in December was “to frighten the pants off everyone” with a declaration of a new strain of COVID-19, known as the Alpha or Kent variant.
In a conversation between Mr Hancock and Mr Poole on Dec 13th, the pair discussed how to survive the coming backlash and storm. On the day, there were 18,409 cases of Covid recorded and 410 deaths. Five days later, on Dec 18th, Mr. Johnson would scrap his planned five-day Christmas amnesty in an about turn.
Every time I think I have hit peak anger with this ongoing saga, the messages reveal a new low.
The bizarre power given to chancers “Slackie and Lee” (James Slack and Lee Cain in the Downing St comms team) to dictate policy; Simon Case’s nauseating glee at imposing petty restrictions, and his characterisation of the desire to retain basic privacy around one’s contact details as “pure Conservative ideology”; Boris’s claim that another lockdown would be the “height of absurdity”, before immediately implementing it – I could go on.
But Hancock’s brazen fear tactics, apparently divorced from any kind of scientific evidence, deployed, to use his word, with borderline psychopathic disregard for the impact they would have on people, is the worst finding yet in this already incredibly sordid tale.