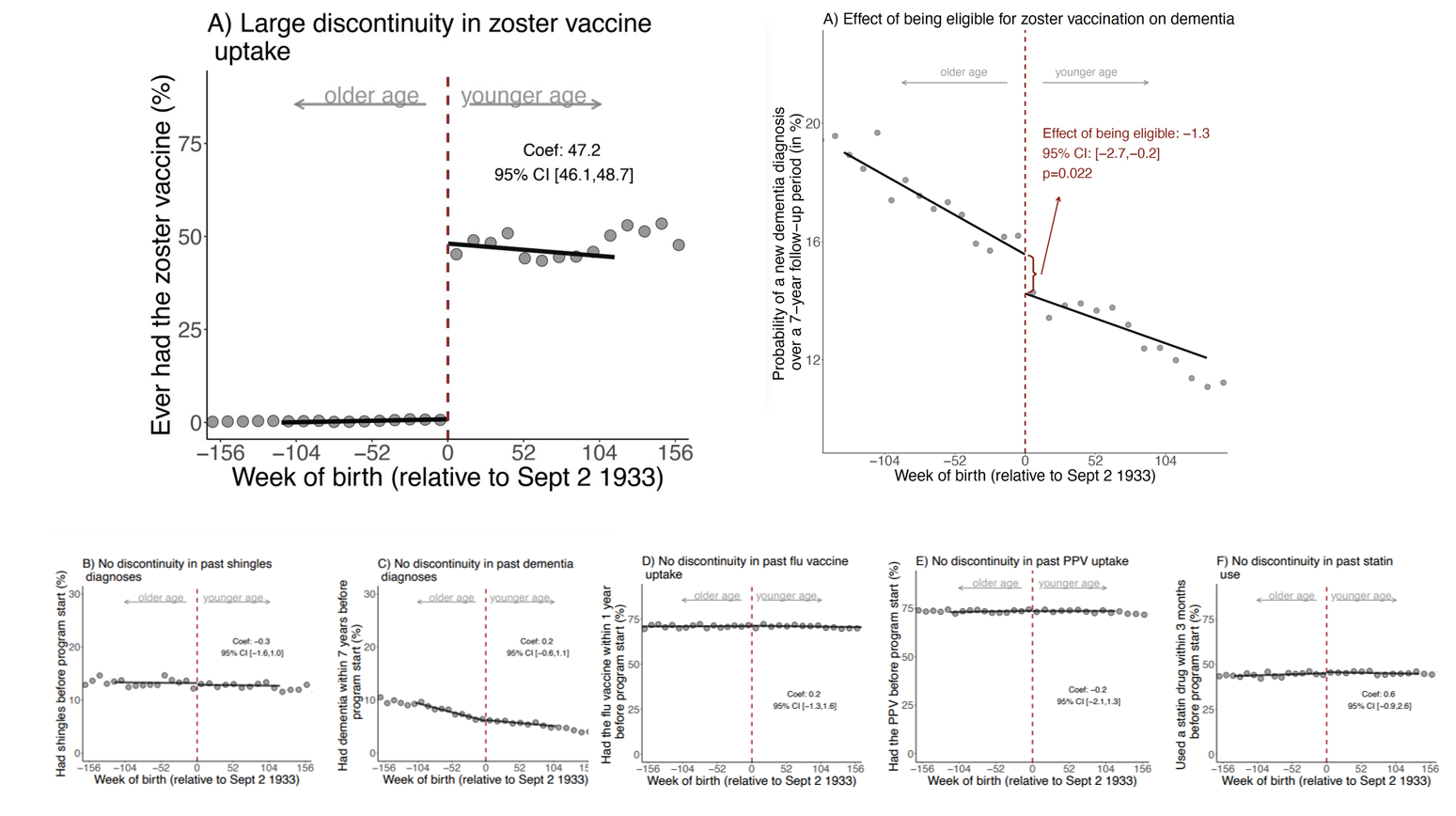
A day before the Pentagon celebrated the June 1 start of Pride Month, top defense leaders were quietly enforcing a militarywide ban on drag performances.
The move became public after Defense Secretary Lloyd Austin and Joint Chiefs of Staff Chairman Gen. Mark Milley stepped in to stop a drag show at Nellis Air Force Base, Nev., which had been scheduled for Thursday.
In enforcing the ban, which stems from a long-standing Defense Department policy that has not always been applied, critics say the Pentagon appears to have acquiesced to GOP pressure in recent months to cancel those performances at military installations.
Republican lawmakers, in hearings and letters to the Defense Department, have raged over drag shows and diversity programs, which they claim hurt recruiting efforts and compromise warfighting.
LGBTQ rights groups have slammed the apparent shift from the Pentagon, noting the U.S. has a long history of supporting drag shows going back to at least World War I.
Kelley Robinson, president of the Human Rights Campaign, said Austin has been “unwavering” in supporting LGBTQ groups, but by banning drag shows, he “chose to side with the politics of fear and discrimination peddled by extreme members of Congress.”
The GOP has beamed with approval over the news this week and appears emboldened to press the issue further.
Rep. Mike Johnson (R-La.) said he was “glad the Pentagon has made clear this shouldn’t be allowed.”
“China is building hypersonic missiles while the U.S. Secretary of Defense is being forced to explain why there are drag shows on our military bases,” Johnson said in a statement. “The fact that these events were scheduled once again calls into question the priorities of our military’s leadership.”
The enforcement first came to light after NBC News reported on the drag show cancellation at Nellis Air Force Base. The Air Force pointed to recent congressional testimony from Austin in explaining the scrapped plans — the defense chief said drag events are “not something that the department funds.”
Citing a policy on standards of conduct and ethics regulation, Pentagon deputy press secretary Sabrina Singh said this week “certain criteria” must be met for nongovernment individuals and organizations using Defense Department facilities and equipment.
“Hosting these types of events in federally funded facilities is inconsistent with regulations regarding the use of DoD resources,” she said in a statement regarding the Nellis Air Force Base drag show cancellation.
“We are proud to serve alongside any and every young American who takes the oath that puts their life on the line in defense of our country. Service members and their families are often involved in a host of special interest activities related to their personal hobbies, beliefs, and backgrounds.”
A White House official referred questions about the policy to the Pentagon.
Robinson of the Human Rights Campaign noted the Pentagon’s move comes as LGBTQ Americans are facing growing threats and fears of violence.
“For decades, our community has fought for our right to exist without shame or exception, yet the Secretary’s decision to ban an event that has happened in prior years reinforces false tropes about LGBTQ+ culture,” Robinson said in a statement.
“At a time when we are under attack, the Pentagon is ceding to extremist forces focused on taking away our rights —leaders responsible for national defense ought to do better.
The event’s cancellation appears to be the first time senior Defense officials have enforced the policy, as Nellis AFB hosted a Pride Month drag show in June 2021.
Yeoman 2nd Class Joshua Kelley, a former Navy digital ambassador, performed in drag under the name “Harpy Daniels” while serving on the aircraft carrier USS Ronald Reagan from 2016 to 2018. Kelley also received intense backlash from the GOP for appearing as a drag queen.
Other drag shows and drag-themed events have also taken place on military installations across the country in previous years.
When asked why the ban has now been applied, the Pentagon did not respond.
The action seems to stand in contrast to Austin’s official statement to mark the start of Pride Month, released Thursday, in which he said he believes “that the story of America should be one of widening freedom, not deepening discrimination” and vowed to ensure LGBT individuals could continue to serve.
“This Pride Month, we honor the service, commitment, and sacrifice of the LGBTQ+ Service members and personnel who volunteer to defend our country,” Austin states. “Their proud service adds to America’s strength.”
Some GOP lawmakers still aren’t satisfied with the ban on drag shows on military bases.
Rep. Mark Alford (R-Mo.), a frequent critic of diversity, equity and inclusion programs at the Pentagon, said the “decision to end drag shows on military bases is a positive first step” but added he would “not stop until all wokeness is weeded out of our military.”
“Why were they happening in the first place?” Alford said in a statement. “Earlier this year, Secretary Austin came before the House Armed Services Committee and told us that drag shows were not taking place on U.S. military installations. Clearly, that was not true.”
Rep. Matt Gaetz (R-Fla.) also hailed the decision, calling it a “huge victory.”
“Drag shows should not be taking place on military installations with taxpayer dollars PERIOD!” he tweeted.
Gaetz had questioned Austin and Milley about “drag queen story hours” on U.S. military installations in the U.S. and in Germany during a March House Armed Services Committee hearing.
Milley responded to the questioning that he was not aware of the events and wanted to “take a look at those, because I don’t agree with those.”
Austin replied that the event is “not something that the department funds.”
The comments from the nation’s top defense officials were scooped up by the GOP and conservative media.
The testimony at that hearing appears to have marked a shift in the enforcement of ethics and standards of conduct policies for drag shows on military installations.
An Air Force official confirmed to The Hill that “consistent with Secretary Austin’s congressional testimony, the Air Force will not host drag events at its installations or facilities.”
“Commanders have been directed to either cancel or relocate these events to an off-base location.”
The March hearing was followed by a House Republican letter to Austin in May, led by Alford, demanding he “put an end to any drag shows and any ‘drag queen influencers’ performing in our military” and instead focus on “patriotism” and pride in “American greatness.”
“We’re at a time where America’s youth has very little desire to serve and protect our nation,” the House Republicans wrote at the time. “The Navy’s decision to use drag queens as a recruitment tool is outrageous and a disgrace to those who have previously served.”
Defense officials were also facing pressure from the Senate side.
Sen. Steven Daines (R-Mont.) last month introduced legislation to ban Pentagon funding for drag shows on military bases.
Daines had expressed outrage at previous drag show events in Virginia and Nevada as well as a 2021 event in his home state that reportedly included a drag queen story hour for children.
The Pentagon’s retreat from the culture wars this Pride Month also appeared to extend to social media.
The Navy’s official Instagram and Twitter accounts removed Thursday posts celebrating the start of Pride Month.
The Instagram post depicted a fighter jet with the LGBTQ+ flag colors in its wake, but by Friday morning, the image was gone.
In a statement to The Hill, a Navy spokesperson acknowledged the original posts but would not say why or when they were removed.
“The US Navy posted graphics in support of the start of Pride month to honor the service, commitment, and sacrifice of the LGBTQ+ Service members and personnel who volunteer to defend our country,” they said in a statement.
The Modern Military Association of America, the nation’s largest organization of LGBTQ+ service members, military spouses, veterans and their families, said in a statement to The Hill they were “deeply troubled” by the drag ban and were “determining next steps regarding communications with the Pentagon and Biden administration about this issue.”
The group pointed out that drag performances have long been a part of military history, including during WWII, when all-male theater performances were produced with soldiers dressing in drag to impersonate women.
The group said it was “concerned that censorship of LGBTQ-friendly events sends the message to LGBTQ+ members of the armed forces that wearing their preferred clothing or acting in a manner different from the gender assigned to them at birth is unacceptable.”
It noted that nearly 80,000 lesbian, gay and bisexual people were currently in active service, along with another 15,000 transgender people.
“Ensuring our ranks reflect the diversity of the American people is essential to morale and cohesion,” it added.