The Biden administration on Wednesday appealed a federal judge's ruling restricting some agencies and officials from meeting and communicating with social media companies to moderate their content, according to a court filing.
Search This Blog
Wednesday, July 5, 2023
Britain's banks seek government roadmap to tokenise stocks and bonds
Britain's role as a leading financial centre is at stake unless it comes up with a roadmap for digital representations of stocks, bonds, loans and real estate, trade association UK Finance said in a report on Thursday.
So-called tokenised assets can be exchanged on distributed ledger technology (DLT), also used for cryptocurrencies, to keep track of transactions, validate ownership, and dispense with a paper trail.
The report, written with consultants Oliver Wyman, said tokenised issuance is still a fraction of securities issued traditionally on exchanges, with digital bond issuance just 1% of the $20.6 trillion of long-term fixed income issued in 2021.
Issuance in Britain has been minimal compared with places such as Singapore, Hong Kong, France and Germany, and it needs to partner with industry to increase momentum, the report said.
"The public sector should guide innovation by setting out a clear and supportive roadmap for tokenisation and encouraging the public and private sectors to collaborate on needed infrastructure and standards," it said.
"Failure to act will result in the UK losing an opportunity to consolidate its position as a top global financial centre."
There is growing consensus that tokenisation could be transformational for markets, though estimates on the impact vary widely, the report said.
"Industry participants agree that tokenisation will enable heightened liquidity across new and existing asset classes, but opinion is mixed as to which asset classes present the most value," it said.
A new financial services law was approved last week which will launch a platform later this year for testing the use of DLT in market infrastructure.
The finance ministry and Financial Conduct Authority should take urgent action beyond the issuance of digital UK government bonds to encourage experimentation with tokenised securities, the report said.
Secret Service Confirms Cocaine Was Found In West Wing Phone Cubby Hours After Hunter Biden Visit
Update (5:15pm): The puzzle, wrapped in an engima, inside a bag of blow surrounding the "mystery" cocaine found in the White House is drawing in some of the world's most cunning detectives and brilliant minds.
One day after leaked radio intercepts revealed that a "mystery" substance was found over the weekend inside the White House and - after leading to a brief evacuation over hazmat fears - was "cocaine like", the US Secret Service on Wednesday confirmed what everyone already knew - the powdery substance found inside the White House over the weekend is cocaine.
While little new was revealed, secret service spokesman Anthony Guglielmi said that the cocaine was discovered in an area of the West Wing lobby where individuals can store their phones. The lobby is “a heavily traveled area” regularly accessed by both visitors and staff, White House Press Secretary Karine Jean-Pierre said Wednesday.
And now, the hunt for the coke fiend begins.
“The president thinks this is incredibly important to get to the bottom of,” she added, and surely every able-bodied FBI agent is on top of it.
The good news is that since every visitor to the White House is logged and every square inch of the premises is under constant video surveillance, the mystery won't last long...
... unless of course the White House webcam operator was previously in charge of the the Jeffrey Epstein suicide cams all of which "broke" just before the famous pedophile "killed himself."
https://www.zerohedge.com/markets/secret-service-investigating-how-cocaine-got-white-house
Patients Squeezed in Fight Over Who Gets to Bill for Pricey Infusion Drugs
Health insurers and medical providers are battling over who should supply high-cost infusion drugs for patients, with the tussle over profits now spilling into statehouses across the country.
The issue is that some insurers are bypassing hospital pharmacies and physician offices and instead sending more complex drugs through third-party pharmacies. Those pharmacies then send the medications directly to the medical provider or facility for outpatient infusing, which is called "white bagging," or, more rarely, to patients, in what is called "brown bagging." That shifts who gets to buy and bill for these complex medications, including pricey chemotherapy drugs.
Insurers say the policies are needed because hospital markups are too high. But hospitals argue that adding an intermediary results in unnecessary risks and delays, and they say some insurers have their own or affiliated pharmacy companies, creating financial motives for controlling the source of the medications. The patients, meanwhile, are left to deal with the red tape.
Paula Bruton Shepard in Bolivar, Missouri, is among those caught in the middle. Flares of lupus, an autoimmune disease, rob Shepard of her mobility by attacking her joints. She relies on monthly infusions to treat her symptoms. But at times, she said, her treatments were delayed due to UnitedHealthcare's white bagging infusion policy. And interruptions to her treatments exacerbated her symptoms.
"I once had to use a toilet lift and it was kind of demoralizing to say, 'I'm a 50-year-old woman and I have to use a toilet lift,' " Shepard said of the medication delays.
This is a tug of war over profits between insurers and medical providers, said Ge Bai, a professor of accounting and health policy at Johns Hopkins University. While insurers claim the arrangement reduces costs, she said, that doesn't mean insurers pass along savings to patients.
"I don't think we should have more sympathy toward one party or the other," Bai said. "Nobody is better than the other. They're all trying to make money."
The savings from white bagging can be significant for expensive infusion drugs, according to a report from the Massachusetts Health Policy Commission. For example, Remicade, used to treat a variety of inflammatory diseases, including Crohn's, cost on average $1,106 per unit in 2015 under hospitals' traditional buy-and-bill system, the commission found in its review of state claims data. That same drug cost an average of $975 per unit under white bagging, a 12% savings.
But the report also found patients, on average, faced higher cost sharing — what they are responsible for paying — for Remicade and other drugs when white bagging was used. While some patients had only modest increases to their costs under the policy, such as $12 more for a medication, the review found it could mean much greater cost sharing for some patients, such as those on Medicare.
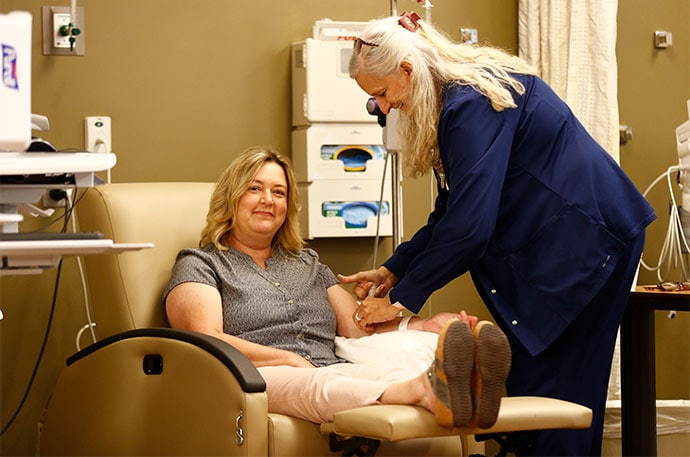
Paula Bruton Shepard had difficulty getting monthly infusions to treat her lupus. Her local hospital, in Bolivar, Missouri, had the drug. But her insurer required it to be sent through a separate pharmacy, which she says led to delays that caused her symptoms to worsen.
At Citizens Memorial Hospital in rural Bolivar, more than 1 in 4 patients who receive regular infusions are being forced to use an outside pharmacy, said Mariah Hollabaugh, the hospital's pharmacy director. Shepard was among them.
Even if the hospital has the exact drug on the shelf, patients must wait for a separate shipment, Hollabaugh said, potentially interrupting care. Their shipped drugs may sometimes be unusable when the doctor needs to change the dosage. Or the medicine comes in a nondescript package that doesn't get immediately flagged for the pharmacy, potentially subjecting the drugs to damaging temperature fluctuations. For patients, that can mean delays in care.
"They're in pain, they're uncomfortable," Hollabaugh said. "They may be having symptoms that don't allow them to go to work."
Siteman Cancer Center, led by physicians from Washington University School of Medicine in St. Louis, has confronted the same issue. But the cancer center's size has helped it largely avoid such insurer policies.
John DiPersio, a Siteman oncologist and researcher who led the university's oncology division for more than two decades, said Siteman reluctantly allows white bagging for simple injectables but refuses to accept it for complicated chemotherapies. It does not accept brown bagging. Occasionally, he said, that means turning patients away.
"You're talking about cancer patients that are getting life-threatening treatments," DiPersio said, referring to the dangers of chemo drugs, which he said can be fatal if used improperly. "It doesn't make any sense to me. It's all stupid. It's all lunacy."
At least 21 states, including Missouri, introduced some form of white or brown bagging legislation during the most recent legislative session, according to the American Society of Health-System Pharmacists. And in the past two years, the trade group said, at least 13 states have already enacted restrictions on white bagging, including Arkansas, Louisiana, and Virginia.
ASHP has created model legislation to limit insurers from requiring the practices as a condition of coverage.
"This is a major issue," said Tom Kraus, a vice president at the trade group. "We see this as central to our ability to coordinate patient care."
At the heart of the tension is an often-litigated federal program that allows certain hospitals and the clinics they own to purchase drugs at deep discounts. The 340B program, named for a section of the law that created it, allows hospitals to buy certain drugs for much less — sometimes for a total cost of a single penny — than what they are later paid for those drugs. Hospitals are not required to pass along 340B savings to patients.
The program was intended to help hospitals spread scarce resources further to treat patients in poor and vulnerable communities, but it has morphed into a means of enriching hospitals and their affiliated clinics, researchers said in a 2014 Health Affairs report. Hollabaugh said many rural facilities such as Citizens rely on the revenue generated from the 340B drugs to subsidize infusions that have no profit margin.
The number of participating hospitals and their affiliated outpatient clinics has increased significantly since the 340B program was created in 1992. More than 2,600 of the nation's roughly 6,100 hospitals were participating in the 340B program as of January 2023. That gives them access to discounts that can knock off as much as 50% of a drug's cost, according to the Health Resources & Services Administration, which oversees the program.
The insurance industry argues that hospital markups, especially when made on top of those discounts, have gotten out of control.
"The fact is, people got greedy," Shannon Cooper, a lobbyist for Blue Cross and Blue Shield of Kansas City, said during a Missouri state Senate hearing in March.
Markups are not unique to 340B hospitals, said Sean Dickson, who helps lead pharmaceutical policy for AHIP, a trade group formerly known as America's Health Insurance Plans. The markups thrusted on commercial plans are "widely out of line" with what Medicare will pay, he said, and that is driving up costs without providing additional value.
Legislation that targets white bagging hinders an insurer's ability to rein in such costs, Dickson said, especially when an area lacks competition.
"What we're really trying to focus on here is putting pressure on those markups that are not related to cost or safety," Dickson said.
Anthem Blue Cross and Blue Shield lobbyist David Smith testified during the March hearing in Missouri that even the idea of white bagging elicited a quick response and that almost every major hospital system in the state said they would drop their prices and come back to the negotiation table.
For now, Citizens Memorial Hospital and other Missouri medical facilities will have to continue to tango with the insurers: Legislation to limit white and brown bagging did not pass during the Missouri General Assembly's recent session.
Shepard, though, won't need such legislation.
UnitedHealthcare had been sending her lupus infusion through other pharmacies on and off since 2021, unwilling to cover the drugs if they came from Citizens' in-house pharmacy. Shepard had to authorize each shipment before it was sent. If she missed the monthly call, she said, it was a "bureaucratic mess" trying to get the medication shipped.
"We are driving unnecessary costs out of the health care system to help make care more affordable, while also maintaining drug safety, effectiveness and quality of care," UnitedHealthcare spokesperson Tony Marusic wrote.
But after KFF Health News inquired about Shepard's case, Marusic said UnitedHealthcare stopped white bagging Shepard's medication to "prevent potential delays in shipping." And during her latest infusion in June, her hospital was again able to supply Shepard's medication directly.
"I'm just so relieved," Shepard said. "I don't have to take phone calls. I don't have to reply to emails. I just show up."
https://www.medscape.com/s/viewarticle/994021
FDA Approves Abbott First Leadless Dual-Chamber Pacing System
The US Food and Drug Administration (FDA) has approved "the world's first" leadless dual-chamber pacing system, one based in part on an already-approved leadless single-chamber device, Abbott has announced.
The company's AVEIR DR leadless pacing system consists of two percutaneously implanted devices, the single-chamber AVEIR VR leadless pacemaker, implanted within the right ventricle, and the novel AVEIR AR single-chamber pacemaker for implantation in the right atrium.
The AVEIR DR system relies on proprietary wireless technology to provide bi-directional, beat-to-beat communication between its two components to achieve dual-chamber synchronization, the company stated in a press release on the approval.
The system also provides real-time pacing analysis, Abbott said, allowing clinicians to assess proper device placement during the procedure and before implantation. The system is designed to be easily removed if the patient's pacing needs evolve or its battery needs replacing.
Experienced operators achieved a 98% implantation success rate using the AVIER DR system in a 300-patient study conducted at 55 sites in Canada, Europe, and the United States, as reported previously by theheart.org | Medscape Cardiology. In that study, 63% of the patients had sinus-node dysfunction and 33% had AV block as their primary dual-chamber pacing indication.
The system exceeded its predefined safety and performance goals, providing AV-synchronous pacing in 97% of patients for at least 3 months, it was reported in May at the Heart Rhythm Society 2023 Scientific Sessions and in a simultaneous publication in the New England Journal of Medicine.
"Modern medicine has been filled with technological achievements that fundamentally changed how doctors approach patient care, and now we can officially add dual chamber leadless pacing to that list of achievements," co-author Vivek Reddy, MD, director of cardiac arrhythmia services for Mount Sinai Hospital and the Mount Sinai Health System, New York City, said in the press release.
'Stimulants for ADHD in Childhood Not Linked to Substance Use Later On'
For children with attention deficit-hyperactivity disorder (ADHD), use of prescription stimulant medication to manage their symptoms was not associated with later substance use, according to a longitudinal analysis.
Using a causal analytic method to account for age and other time-varying characteristics, including household income, behavior problems, and parental support, there was no evidence showing that current (B range -0.62 to 0.34) or prior stimulant treatment (B range -0.06 to 0.70) or their interaction (B range -0.49 to 0.86) were associated with substance use in adulthood, reported Brooke Molina, PhD, of the University of Pittsburgh, and co-authors.
Models adjusting for confounding by demographic, clinical, and familial factors also showed no evidence that more years of stimulant treatment (B range -0.003 to 0.04) or continuous, uninterrupted stimulant treatment (B range -0.25 to -0.03) were associated with later substance use, including heavy drinking, marijuana use, and daily cigarette smoking, among other substance uses, they noted in JAMA Psychiatry
Similar results were also observed for substance use disorder.
"Parents and providers can be reassured that we found no evidence of increased risk of substance use or substance use disorder when children are prescribed stimulant medications for ADHD," Molina told MedPage Today.
"Because this study took into account the many reasons that medication and substance use may seem connected, it extended the literature base in an important way," she added. "For example, just age alone is associated with decreased use of prescribed stimulant medication and increased substance use over time, which can make them correlated for reasons besides one causing the other."
Molina and co-authors used prospective longitudinal data from the randomized Multimodal Treatment Study of ADHD (MTA), which followed patients with ADHD over a 16-year period from childhood through adolescence into early adulthood.
"We know, from multiple studies, including several others from the MTA, that ADHD is a risk factor for substance use disorders," Molina said. In their introduction, the authors noted that "early exposure to stimulants may cause neurobiological and behavioral sensitization to other drugs and thus increase the risk for harmful substance use."
However, "our confidence is now increased that prescribing stimulants in childhood does not contribute to this problem," Molina said.
"Parents and providers should remember that ADHD is a chronic condition that needs treatment throughout life. Stimulant medication, along with other treatments like parent and teacher training and individual and group therapies for adults, should regularly be considered to maximize the best outcomes, including reducing risk of substance use disorder," she continued.
MTA was conducted at six sites in the U.S. and one site in Canada. It was initially designed to be a 14-month randomized clinical trial of medication and behavior therapy for ADHD, but the study eventually transitioned into a longitudinal observational study. Participants were enrolled from 1994 through 1996.
In total, the researchers included 579 children (mean age at baseline 8.5). Participants were mostly male (80%) and white (61%). Children ages 7 to 9 with DSM-IV combined-type ADHD were repeatedly assessed until a mean age of 25 years.
Stimulant treatment for ADHD was measured prospectively from baseline for 16 years, for a total of 10 assessments, first using parent report, and then young adult report.
The proportion of adolescents using stimulant medication decreased through adolescence, from close to 60% at the 2- and 3-year assessments to 7.2% on average in early adulthood.
Mean percentages across the 12-, 14-, and 16-year follow-up assessments were 36.5% for daily smoking, 29.6% for marijuana use at least weekly, 21.1% for heavy drinking at least weekly, and 6.2% for other substance use at least monthly.
Limitations included a lack of medical records to verify medication history, Molina and team noted. Furthermore, they said, though the study sample included females (20%) and African Americans (20%) and Hispanics (8%), the study was "insufficiently powered for a strong test of moderation by sex, race, or ethnicity."
Disclosures
The Multimodal Treatment Study of Children with ADHD was a National Institute of Mental Health (NIMH) cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and finally under a National Institute on Drug Abuse contract followed by a data analysis grant.
Molina reported grants from the NIMH and National Institute on Drug Abuse during the conduct of the study. Co-authors reported multiple relationships with various government entities, foundations, and pharmaceutical companies.
Primary Source
JAMA Psychiatry
Source Reference: opens in a new tab or windowMolina BSG, et al "Association between stimulant treatment and substance use through adolescence into early adulthood" JAMA Psychiatry 2023; DOI: 10.1001/jamapsychiatry.2023.2157.
Patients, Doctors Fear AI in Medicine
The sanctity of the doctor-patient relationship has always been a cornerstone of modern medicine. It's a relationship rooted in trust, confidentiality, and mutual understanding.
Yet, with the advent of generative artificial intelligence (AI) technologies like ChatGPTopens in a new tab or window, apprehension is growing among doctors and patients. Physicians fear these tools will undermine (or replace) them. Patients worry the personal touch of their healthcare providers will be replaced by zeros and ones.
A recent Pew Research Pollopens in a new tab or window found that most Americans feel this way. Sixty percent of patients surveyed said they feared their healthcare provider would rely too much on AI to diagnose disease and recommend treatments, and 57% said they worried that AI will erode the connection they have with their healthcare provider.
As millions of Americans begin to navigate the technological revolution of medicine, a little technophobia is understandable. But fear shouldn't overshadow the valid reasons for optimism.
ChatGPT and similar technologies have the potential to strengthen, rather than compromise, medical care in the U.S. If trained properly and used wisely, AI can rekindle, not wreck, the doctor-patient relationship.
To understand the upside, consider the No. 1 fear patients express about AI in healthcare: the risk that their doctor will depend too much on it.
This isn't a new type of anxiety. According to a 2021 opinion pollopens in a new tab or window out of Penn State, taken more than a year before the rollout of ChatGPT, 77% of Americans said they believe society relies too much on technology to succeed.
But when it comes to the doctor-AI relationship, there's little risk of physicians leaning too hard on ChatGPT to the detriment of patients. More likely, AI will merely bolster the doctor's decision-making.
Already, doctors and their teams are using generative AI tools like ChatGPT and Med-PaLM 2opens in a new tab or window when seeking a second opinion. In the future, data-rich AI models will arm doctors with a diagnostic support system that can minimize medical errors and maximize patient safety.
At top academic institutions, educators are gearing up to teach medical students and residents how to use AI safely and effectively. Educators agree that chatbots, when used responsibly, can accelerate and deepen medical learning
opens in a new tab or window -- while also helping students avoid the well-documented pitfalls of information overload and burnoutopens in a new tab or window. Meanwhile, Congress has begun hearingsopens in a new tab or window on AI with the intent to protect Americans from harm.
For the foreseeable future, we can expect doctors to remain accountable for all medical decisions while turning to ever-more reliable AI sources for a helpful boost.
In addition to fears that AI will damage the doctor-patient relationship, another common concern is that AI, like any new technology, might glitch, generating inaccurate and potentially deadly adviceopens in a new tab or window. This, too, is a valid concern. Today's generative AI tools can't be used in medicine without a physician's oversight. But as the tech becomes more dependable, it has the potential to fill a huge healthcare void.
Imagine, for example, your child awakens at midnight with a 103° fever. The doctor's office is closed and, should you call anyway, a recorded message will tell you to "dial 911 in case of an emergency." This leaves you as a parent with two options: wait until morning to telephone your family doctor and hope your child doesn't die. Or race to the emergency department, where you'll likely wait hours to be seen and get charged 12 times moreopens in a new tab or window than at your physician's office.
Future generations of AI will likely give parents another option. AI technologies are doubling in power
opens in a new tab or window and performance every 3opens in a new tab or window to 6opens in a new tab or window months, which means next-gen AI should have no trouble learning to ask the same questions as medical professionals who work in 24/7 clinical call centers. And AI will be trained to follow the same expert protocols, too. Once that happens, tools like ChatGPT will be able to dispense safe, reliable, and immediate medical advice -- day or night.
And when it comes to concerns that AI will depersonalize doctor visits, patients should consider a likelier possibility. Most doctors spend 10 to 20 hours a weekopens in a new tab or window on administrative duties like filling out insurance forms and entering patient data into medical records. These mundane tasks not only contribute to rising rates of burnout in medicine, but also consume precious time that physicians could be spending with patients.
By delegating these jobs to AI (using an existing suite of voice recognition, transcription, and automation tools), your physician can focus more on you and less on the computer that, today, sits between the two of you.
Another set of patient concerns relates to privacy and security.
AI systems depend on massive data sets. In medicine, these databases may one day contain the totality of your medical information and would be a tempting target for cyber criminals. But it will be much harder for evildoers to breach those files than patients might think. That's because companies like Microsoft and Google are helping develop some of medicine's leading AI products.
Tech giants couldn't survive without sufficient public trust. Therefore, they'll have a strong financial incentive to keep our medical information as well protected as our passwords, credit card information, and browsing history. In our digital world, security and privacy risks are ever present. But those risks will be no greater with medical AI tools than with other technologies we use comfortably today.
Finally, the emergence of AI in medicine will raise an array of ethical questions. Who's responsible when AI makes a medical mistake? How will we ensure that all people have equitable access to AI-based healthcare services? These are complex issues that will require thoughtful dialogue among ethicists, physicians, patients, technologists, and policymakers.
Congress will need to do its part to define guidelines and regulations that govern the ethical use of AI in healthcare, protecting patient interests while fostering innovation. But as we compare the potential risks that AI poses against the potential health benefits it offers, all of us -- patients and medical professionals -- should feel optimistic. Generative AI will improve the practice of medicine and our personal health.
Robert Pearl, MD, is a professor at Stanford's School of Medicine and Graduate School of Business in California and the former longtime CEO at the Permanente Medical Group (Kaiser Permanente).
