Novo Nordisk said on Thursday treating obesity with its weight-loss drug Wegovy as a precursor to type 2 diabetes was a priority for the company, continuing the Danish drugmaker's shift away from its century-long focus on insulin.
The company on Thursday reported record sales and operating profits for the third quarter driven by demand for Wegovy, even as the company cautioned shortages will continue in the short to medium term.
Wegovy sales totalled 9.6 billion Danish crowns ($1.36 billion) between July and September, 28% higher than the previous quarter and up eight-fold from the same period last year.
Insulin sales stood at 11.3 billion Danish crowns, almost unchanged from the previous quarter and down 12% from a year before, continuing a years-long trend. A drop in the U.S. was driven by lower realised prices and declining volume.
On a media call after the results announcement, Novo CEO Lars Fruergaard Jorgensen noted the company's 100-year history in diabetes, as one of the first companies to bring insulin to market.
Over the years type 2 diabetes has become the "dominating focus", he said, with many people who develop that type of diabetes suffering from weight issues.
Over time, he said, broader use of Wegovy could lead to fewer people with type 2 diabetes needing to take insulin, which he said would be a "very positive outcome" for society.
Novo's diabetes drug Ozempic and Wegovy are from the class called GLP-1 receptor agonists, and both contain the active ingredient semaglutide, originally developed to help control blood sugar in patients with diabetes.
Ozempic is approved for treatment of type 2 diabetes, while Wegovy is approved for weight loss.
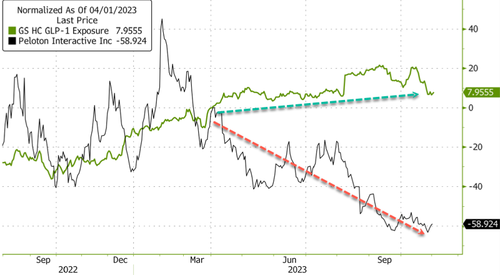
Doctors have told Reuters that patients taking GLP-1s require less insulin. Analysts say that could over time further diminish insulin sales.
Jorgensen also referred to other health problems associated with obesity, such as heart disease, and mentioned the company's large study released in August that showed Wegovy had a clear cardiovascular benefit.
He said the company would continue to pursue other uses for Wegovy, and whether semaglutide could be used in combination with other agents to treat Alzheimer's due to its anti-inflammatory properties.
https://finance.yahoo.com/news/novo-nordisk-obesity-drugs-priority-103101203.html