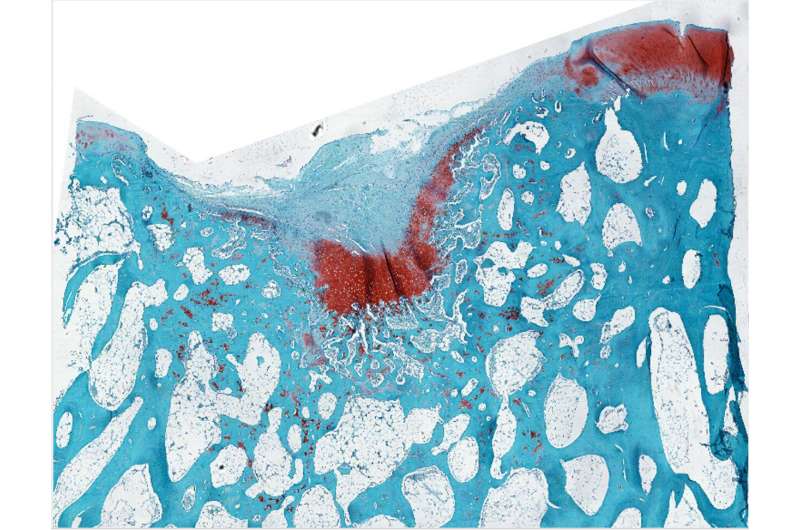
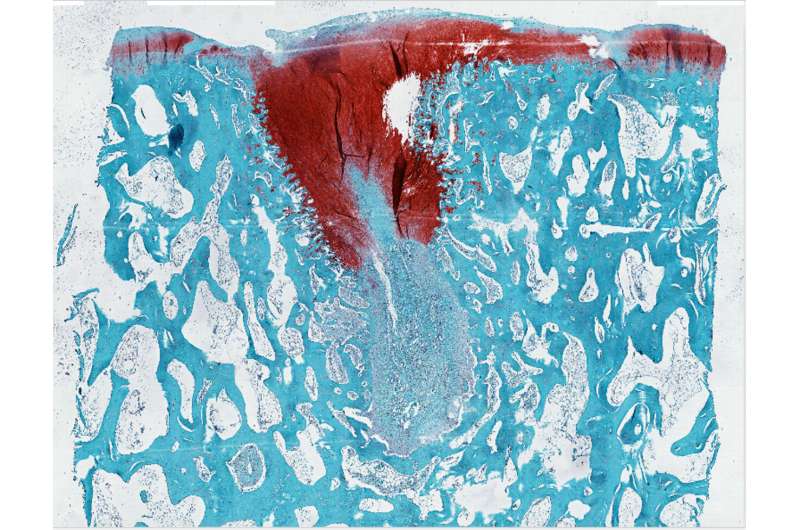
Multiple sclerosis (MS) degrades the protective insulation around nerve cells, leaving their axons, which carry electrical impulses, exposed like bare wires. This can cause devastating problems with movement, balance and vision; and without treatment, it can lead to paralysis, loss of independence and a shortened lifespan.
Now, scientists at UC San Francisco and Contineum Therapeutics have developed a drug that spurs the body to replace the lost insulation, which is called myelin. If it works in people, it could be a way to reverse the damage caused by the disease.
The new therapy, called PIPE-307, targets an elusive receptor on certain cells in the brain that prompts them to mature into myelin-producing oligodendrocytes. Once the receptor is blocked, the oligodendrocytes spring into action, wrapping themselves around the axons to form a new myelin sheath.
It was crucial to prove that the receptor, known as M1R, was present on the cells that can repair damaged fibers. Contineum scientist and first author Michael Poon, Ph.D., figured this out using a toxin found in green mamba snake venom.
The work, which appears Aug. 2 in Proceedings of the National Academy of Sciences, caps a decade of work by UCSF scientists Jonah Chan, Ph.D., and Ari Green, MD. Chan led the team to discover in 2014 that an obscure antihistamine known as clemastine could induce remyelination, which no one knew was possible.
"Ten years ago, we discovered one way that the body can regenerate its myelin in response to the right molecular signal, winding back the consequences of MS," said Chan, a Debbie and Andy Rachleff Distinguished Professor of Neurology at UCSF and senior author of the paper. "By carefully studying the biology of remyelination, we've developed a precise therapy to activate it—the first of a new class of MS therapies."
A dirty drug creates a clean opening
The original breakthrough came when Chan invented a method to screen drugs for their ability to instigate remyelination. The screen identified a group of drugs, including clemastine, that had one thing in common: They blocked muscarinic receptors.
Clemastine's benefits begin with its effect on oligodendrocyte precursor cells (OPCs). These cells stay dormant in the brain and spinal cord until they sense injured tissue. Then they move in and give rise to oligodendrocytes, which produce myelin.
For some reason, during MS, OPCs gather around decaying myelin but fail to rebuild it. Chan figured out that clemastine activated OPCs by blocking muscarinic receptors, enabling the OPCs to mature into myelin-producing oligodendrocytes.
Nerves and their myelin are notoriously hard to repair, whether due to MS, dementia or other injury. Green and Chan carried out a trial of clemastine in patients with MS, and it was a success—the first time that a drug showed the capacity to restore the myelin lost in MS. Despite being safe to use, however, clemastine was only modestly effective.
"Clemastine is not a targeted drug, affecting several different pathways in the body," said Green, Chief of the Division of Neuroimmunology and Glial Biology in the UCSF Department of Neurology and co-author of the paper. "But from the get-go, we saw that its pharmacology with muscarinic receptors could point us toward the next generation of restorative therapies in MS."
A snake venom toxin illuminates the right target
The researchers continued using clemastine to understand the curative potential of regenerating myelin in MS. They developed a series of tools to monitor remyelination, both in animal models of MS and in MS patients, showing that the benefits seen with clemastine came from remyelination—and pointing the way for how new drugs should be tested and evaluated.
They also found that clemastine's benefits came from blocking just one of the five muscarinic receptors, M1R, but the effect on M1R was middling, and the drug also affected the other receptors. The ideal drug would need to zero in on M1R.
At this point, the UCSF scientists needed an industry partner to advance the project. Ultimately, Contineum Therapeutics (then known as Pipeline Therapeutics) was formed to take a meticulous approach to creating that ideal drug. Chan and Green helped the company confirm that M1R was the right target for a remyelinating drug, and then make a drug that blocked it exclusively.
Poon, a biologist at Contineum, realized that MT7, a toxin found in the venom of the deadly green mamba snake, could reveal exactly where M1R was in the brain.
"We needed to prove, beyond doubt, that M1R was present in OPCs that were near the damage caused by MS," Poon said. "MT7, which is exquisitely selective for M1R, fit the bill."
Poon used MT7 to engineer a molecular label for M1R that revealed rings of OPCs gathering around damage in a mouse model of MS and in human MS tissue.
Developing a clinic-ready drug
A team of medicinal chemists at Contineum, led by Austin Chen, Ph.D., then got to work on the drug that Chan and Green envisioned, designing PIPE-307 to potently block M1R and get into the brain.
The researchers tested the effects of the new drug on OPCs grown in petri dishes and the animal models of MS using Chan's and Green's methods for tracking remyelination. PIPE-307 blocked the M1R receptor much better than clemastine; prompted OPCs to mature into oligodendrocytes and begin myelinating nearby axons; and it crossed the blood-brain barrier.
But most tellingly, it reversed the degradation seen in a mouse model of MS.
"A drug might seem to work in these abstract scenarios, affecting the right receptor or cell, but the key finding was actual recovery of nervous system function," Chan said.
In 2021, PIPE-307 passed a Phase I clinical trial, demonstrating its safety. It is currently being tested in MS patients in Phase II.
If it succeeds, it could transform how MS is treated.
"Every patient we diagnose with MS comes in with some degree of pre-existing injury," Green said. "Now we might have a chance to not just stop their disease, but to also heal."
More information: Michael M. Poon et al, Targeting the muscarinic M1 receptor with a selective, brain-penetrant antagonist to promote remyelination in multiple sclerosis, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2407974121
https://medicalxpress.com/news/2024-08-drug-clock-multiple-sclerosis.html