Target $1060
Search This Blog
Friday, September 13, 2024
Novavax 2024-2025 Formula COVID-19 Vaccine Available at Major Pharmacies
Novavax, Inc. (Nasdaq: NVAX), a global company advancing protein-based vaccines with its Matrix-M™ adjuvant, today announced that doses of the Novavax COVID-19 Vaccine, Adjuvanted (2024-2025 Formula) (NVX-CoV2705) to prevent COVID-19 in individuals aged 12 and older are in stock at major pharmacy retailers across the U.S.
Through agreements with pharmacies, Novavax's updated vaccine is expected to be widely available in locations including, but not limited to, CVS Pharmacy, Rite Aid, Walgreens, Costco, Publix, Sam's Club, Kroger, Meijer, hundreds of other regional grocers and thousands of independent pharmacies. Novavax's updated vaccine received Emergency Use Authorization (EUA) on August 30, 2024 from the U.S. Food and Drug Administration (FDA).
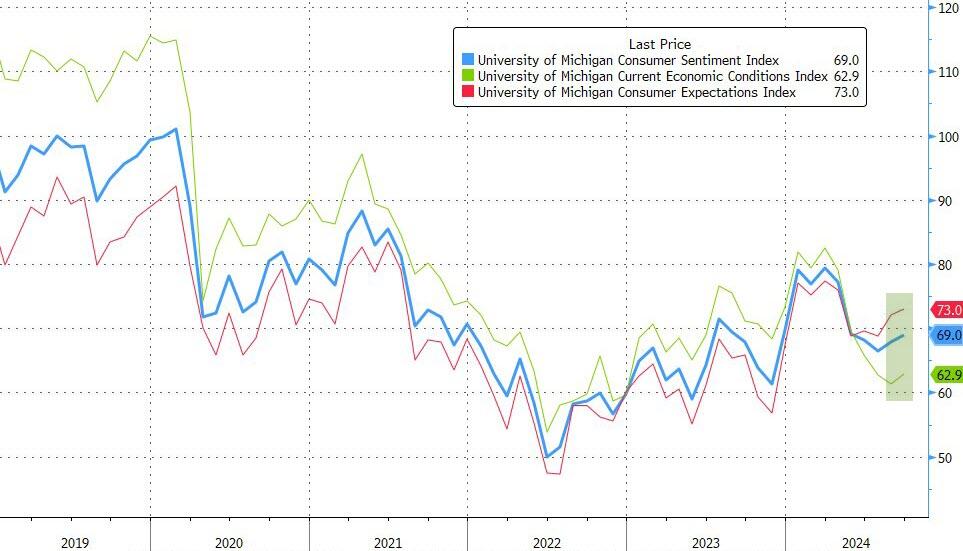
Inflation Expectations Rebound In September As Partisan Gaps In Sentiment Surge
After a hope-filled rebound in August, analysts expect UMich Sentiment in preliminary August data to show further improvements with inflation expectations flat. They were right in their prediction as the headline sentiment beat expectations (69.0 vs 68.5 exp), as did the current conditions and expectations sub-indices.
Source: Bloomberg
The gain was led by an improvement in buying conditions for durables, driven by more favorable prices as perceived by consumers.
Year-ahead expectations for personal finances and the economy both improved as well, despite a modest weakening in views of labor markets.
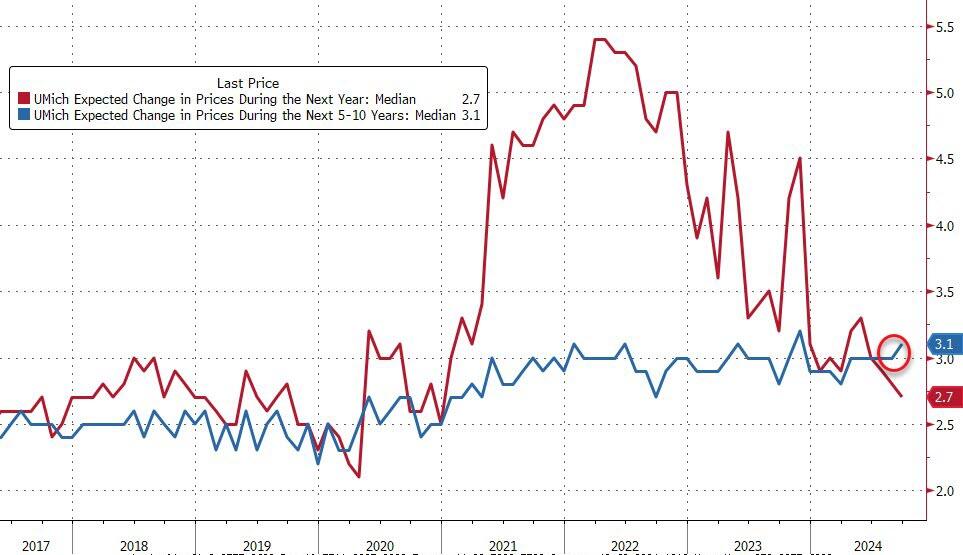
However, more notably, medium-term inflation expectations picked up (while short-dated expectations - which largely reflect oil prices - fell to their lowest since Dec 2020)...
Source: Bloomberg
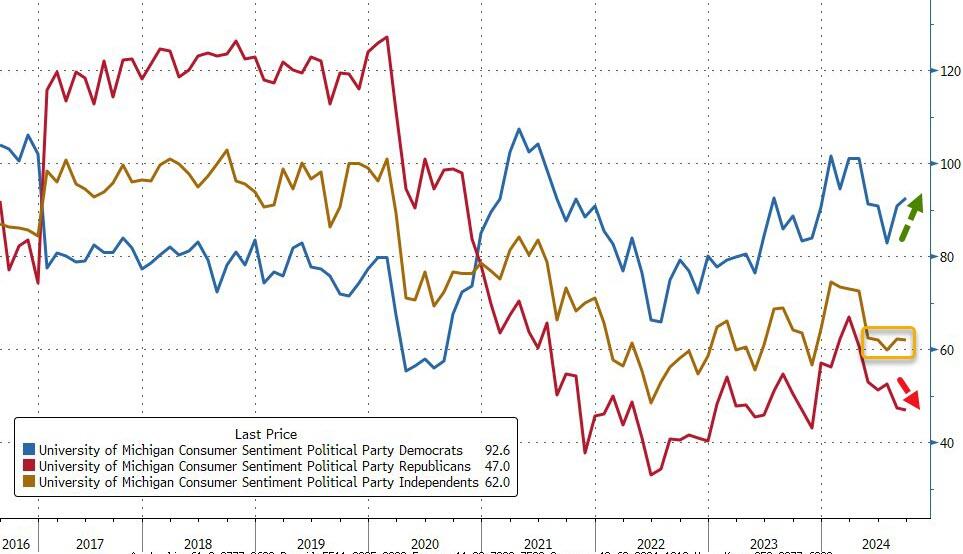
Finally, we note that confidence continued to rise for Democrats, was flat for Independents, and slid further among Republicans...
Source: Bloomberg
Consistent with their divergent views of the implications of a Harris presidency for the economy, partisan gaps in sentiment inched up.
Note that interviews for this release concluded prior to Tuesday’s debate; a more comprehensive look at election expectations will be released next week.
Why Summit Therapeutics Stock Is Soaring Again
Shares of Summit Therapeutics (NASDAQ: SMMT) were soaring 22.5% higher as of 11:18 a.m. ET on Thursday. The gain came after the clinical-stage biopharmaceutical company revealed that it entered into agreements with several biotech institutional and individual accredited investors for the sale of over 10.3 million shares for roughly $235 million in a private placement.
Investors were undoubtedly especially glad to see key insiders agree to buy more Summit Therapeutics stock. Together, co-CEOs Robert W. Duggan and Mahkam Zanganeh, chief operating officer (COO)/CFO Manmeet Soni, chief accounting officer Bhaskar Anand, and board member Jeff Huber are purchasing over 3.48 million shares.
Summit's latest gain came on top of the stock's 85% jump earlier this week. This sizzling momentum is due to the company's overwhelmingly positive results reported on Sept. 8, 2024, from a phase 3 study evaluating ivonescimab as a first-line treatment for advanced non-small cell lung cancer.
In this clinical study, which was conducted in China, ivonescimab reduced the risk of disease progression or death by 49% compared to Merck's blockbuster drug Keytruda. Summit's experimental cancer therapy is the first to beat Keytruda head-to-head in a randomized late-stage clinical trial.
Even with the stellar phase 3 results for ivonescimab, buying a clinical-stage biotech stock is always associated with risks. There are also tremendous expectations for Summit, as reflected by the company's market cap of nearly $19 billion despite having no product on the market. That said, the commercial potential for ivonescimab appears to be huge. I think this stock is one to consider for aggressive investors.
https://finance.yahoo.com/m/f9636552-3123-3d13-89a0-24e79317ee7a/why-summit-therapeutics-stock.html
Tenon Intros Joint Fusion System
~ New SI Joint Fixation Device Offers a 30% Reduction in Implant Size Providing Physicians Choices for Varying Anatomy and Treatment Strategies with the Catamaran Technology ~
~The SE System Can Also Be Utilized with a Manual Drilling Option During Implant Preparation~
Astellas’ Hot Flash Drug Veozah Slapped With Another FDA Warning for Liver Injury
One patient developed elevated liver blood tests after receiving Astellas Pharma’s non-hormonal small molecule blocker, leading the regulator to add a warning to the drug’s label.
The FDA on Thursday added a warning to the label of Astellas Pharma’s hot flash therapy Veozah (fezolinetant), alerting patients and prescribers to a newly documented risk of “rare but serious” liver injury.
The new warning was prompted by a postmarketing review of a patient who was taking Veozah and subsequently saw spikes in liver blood test values. The patient also showed signs of liver injury after being treated for around 40 days, according to the regulator.
Per Veozah’s updated label, prescribers should test their patients’ blood liver markers more frequently, including monthly screens for the first two months after initiating Veozah, followed by additional tests after three, six and nine months. Doctors and prescribers should also conduct hepatic testing before starting patients on Veozah.
In case of signs that could indicate liver problems, patients should stop taking Veozah “immediately” and contact their healthcare professional, according to the FDA. Common symptoms of liver harm include fatigue, jaundice, nausea and vomiting, as well as unusual itching and light-colored stools.
Despite being a hit to Veozah’s safety profile, Thursday’s label seems unlikely to substantially hurt Astellas. Jeffries analysts in a note to investors said that the new warning “strengthened Veozah’s label” and that while the additional testing requirements will put more burden on patients, they “may not have much impact on demand overall.”
Administered orally, Veozah is a non-hormonal small molecule blocker of the NK3 receptor which plays a part in the signaling cascade that results in the release of specific hormones involved in the menstrual cycle. Veozah specifically acts on neurons in the brain’s thermoregulatory centers, allowing it to reduce the frequency and intensity of hot flashes and night sweats. The FDA approved Veozah in May 2023 for women in menopause.
In December 2023, Astellas unveiled more Phase IIIb data for Veozah, touting statistically significant improvements in moderate to severe vasomotor symptoms in patients at 24 weeks compared with placebo. Veozah also reduced sleep disturbance in these patients. At the time, the pharma noted that these findings will help it with reimbursement negotiations in Europe.
Bayer is also advancing a non-hormonal competitor to Veozah. Elinzanetant is a small molecule dual inhibitor of the NK1 and NK3 receptors, which the pharma is also positioning as a treatment for moderate to severe vasomotor symptoms in menopause. Bayer won rights to elinzanetant when it bought KaNDy Therapeutics in August 2020 for $425 million upfront.
In May 2024, Bayer reported data from its pivotal Phase III OASIS 1 and OASIS 2 studies, demonstrating that elinzanetant significantly lowered the frequency of hot flashes, reduced sleep disturbances and improved menopause-related quality of life.
Roche Gains PD-1 Edge on Merck With FDA Nod for Subcutaneous Tecentriq
Tecentriq Hybreza, which combines Roche’s Tecentriq with Halozyme Therapeutics’ Enhanze drug delivery technology, is being touted as the first and only subcutaneous anti-PD-(L)1 cancer immunotherapy.
The FDA on Friday signed off on the subcutaneous formulation of Roche’s PD-1 blocker Tecentriq—which will carry the brand name Tecentriq Hybriza (atezolizumab and hyaluronidase-tqjs) to differentiate itself from the intravenous version—for the treatment of various cancers.
According to Roche, Tecentriq Hybriza is the first and only PD-(L)1 blocker approved for subcutaneous use in the U.S. Tecentriq Hybriza is indicated for all cancers that its intravenous formulation is, including several different types of skin, liver, lung and soft-tissue cancers.
Roche CMO Levi Garraway in a statement said that the approval of Tecentriq Hybriza “builds on the established safety and efficacy of intravenous Tecentriq.” The subcutaneous formulation “can treat patients faster and in more accessible settings” and “offers patients with multiple cancer types and their physicians greater flexibility and choice of treatment administration,” Garraway said.
To enable subcutaneous administration, Tecentriq Hybriza leverages Halozyme Therapeutics’ Enhanze technology, which uses its patented recombinant hyaluronidase PH20 enzyme, which digests hyaluronan proteins beneath the skin to improve fluid flow in the subcutaneous space. In turn, this effect allows large volumes of fluids to be injected under the skin, while also boosting its dispersion and absorption.
Through this technology, Tecentriq Hybriza takes approximately seven minutes to be administered, while an intravenous infusion of Tecentriq usually takes 30 minutes to 60 minutes.
In the Phase Ib/III IMscin001 study, a subcutaneous injection of Tecentriq reached comparable levels of the drug in the blood as its intravenous formulation, with similar safety and efficacy profiles.
Roche also included results from the Phase II IMscin002 study in its application, highlighting that 71% of patients preferred the Hybriza formulation versus intravenous treatment. Convenience—including less time in the clinic, comfort and lower emotional distress—was the most common reason for this preference.
With Friday’s approval, Roche carves out a niche for its PD-1 inhibitor in a market dominated by Merck’s Keytruda (pembrolizumab). In 2023, Tecentriq generated approximately $4.45 billion, while Keytruda easily retained its PD-1 leadership with more than $25 billion in revenue.
Roche has struggled to keep up with Keytruda in cancer, with a late-stage defeat in July 2024. Results from the Phase II/III SKYSCRAPER-06 showed that Tecentriq plus an investigational anti-TIGIT antibody tiragolumab had reduced efficacy in terms of progression-free survival, compared with Keytruda plus chemotherapy.
The trial, which was testing Roche’s investigational regimen for the first-line treatment of non-small cell lung cancer, has since been discontinued.
Merck is hot on Roche’s heels with a subcutaneous formulation of Keytruda in a Phase III study, which is scheduled for completion in late 2026.
https://www.biospace.com/fda/roche-gains-pd-1-edge-on-merck-with-fda-nod-for-subcutaneous-tecentriq