Elon Musk's SpaceX struck a deal with EchoStar Corporation for 50 MHz of exclusive U.S. spectrum and global MSS licenses, a move that supercharges its Starlink Direct to Cell service worldwide.
"This agreement will enable us to develop and deploy our next-generation Starlink Direct to Cell constellation, which will be capable of providing broadband service to cell phones globally," SpaceX wrote in a press release on Monday morning.
Here's the breakdown of the SpaceX–EchoStar spectrum deal:
SpaceX is acquiring 50 MHz of exclusive U.S. S-band spectrum (AWS-4 and PCS-H) plus global MSS licenses from EchoStar.
This spectrum will power next-generation Starlink Direct to Cell satellites, enabling higher bandwidth, optimized 5G protocols, and 100x more capacity than the first generation.
New satellites will be driven by custom SpaceX silicon and phased array antennas, supporting full 5G connectivity comparable to terrestrial LTE.
EchoStar wrote in a press release that SpaceX is paying $8.5 billion in cash and up to $8.5 billion in SpaceX stock for its AWS-4 and H-block spectrum licenses, adding that the proceeds will be used to "retire certain debt obligations and fund EchoStar's continued operations and growth initiatives."
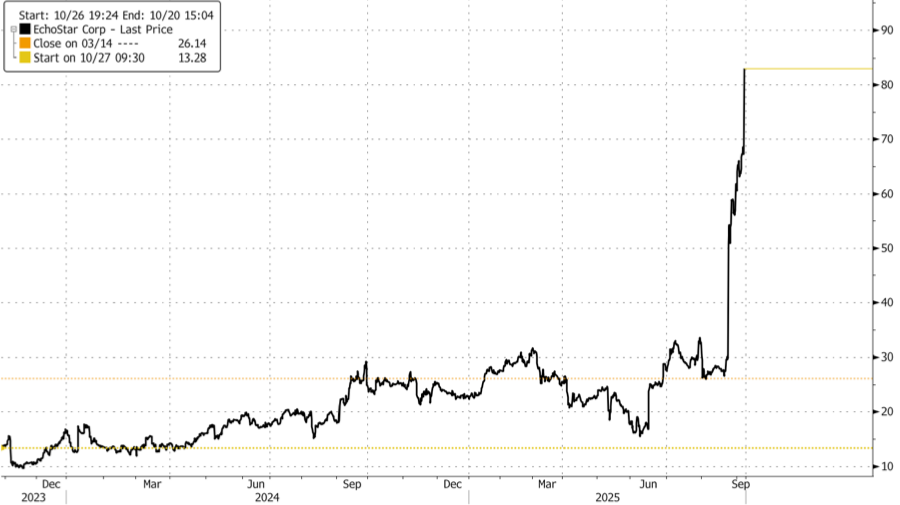
Shares of EchoStar jumped 24% in premarket trading and are up 193% year-to-date as of Friday's close. Short interest stands at 12.37% of the float, or approximately 16.4 million shares, with a 6.1-day average trading volume to cover.
Additional color on Starlink Direct to Cell service:
Service began deployment in January 2024, with early texting and video calling demos on unmodified phones.
Now at 600+ satellites in orbit, the first-gen constellation provides 4G coverage across five continents, serving 6+ million users—the largest 4G footprint on Earth.
Operates at 360 km altitude, using regenerative architecture and laser links to integrate with the 8,000-satellite Starlink backbone.
Starlink operations:
Partnered with T-Mobile, Optus, Telstra, Rogers, KDDI, Kyivstar, and others for global coverage.
Provided life-saving emergency connectivity during U.S. hurricanes, floods, and wildfires, delivering 1.5 million+ connections and hundreds of emergency alerts.
Enabled rescues in remote areas.
In recent weeks, SpaceX's Starship rocket deployed the first batch of mock Starlink satellites in space and tested new heat shield tiles on its plunge through Earth's atmosphere, achieving new milestones in preparation to launch next-gen Direct to Cell satellites with EchoStar spectrum that will expand service, speeds, eliminate dead zones, and extend broadband and IoT connectivity to phones worldwide.
Even though Musk has shown little interest in taking SpaceX public, rumors have circulated about a potential Starlink IPO.
Furthermore, whatever happened to Jeff Bezos' rocket company and space internet company?