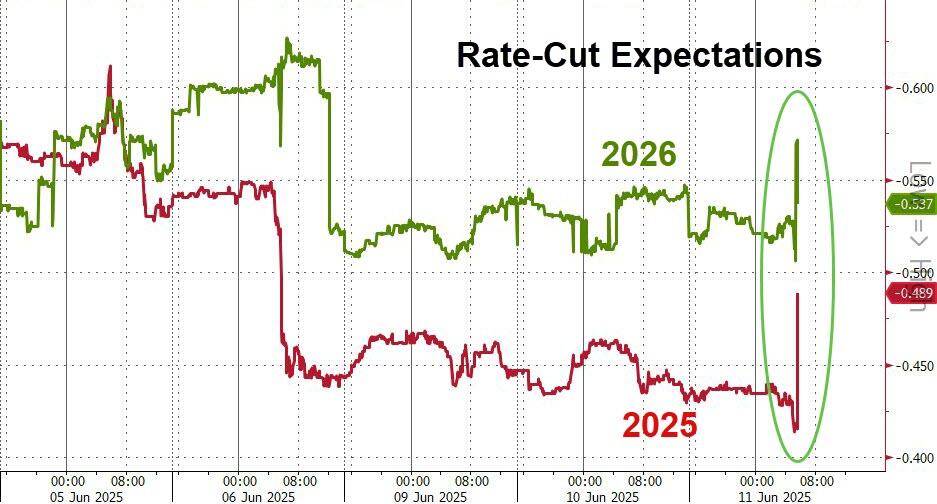
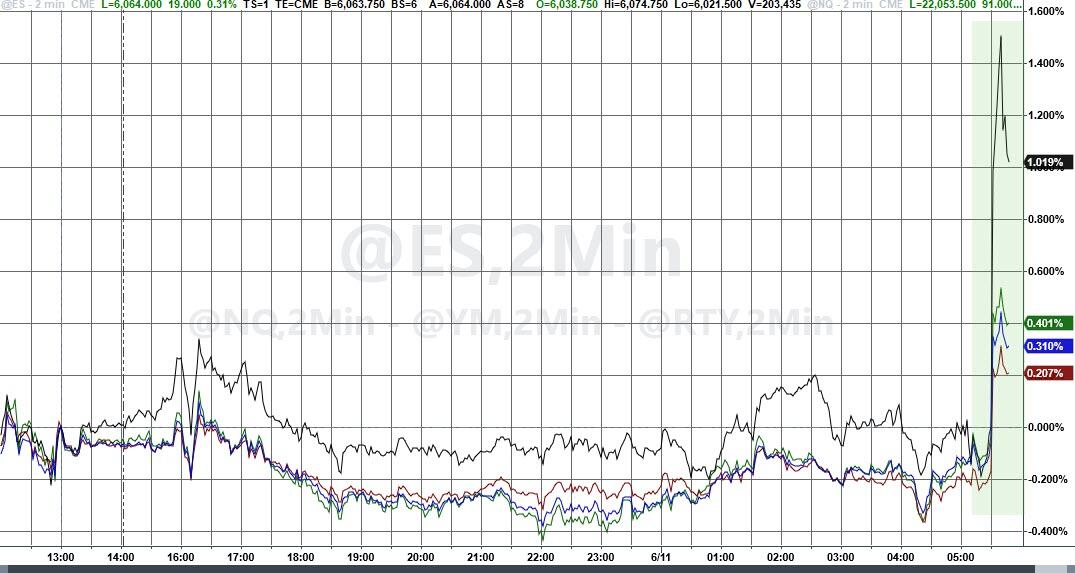
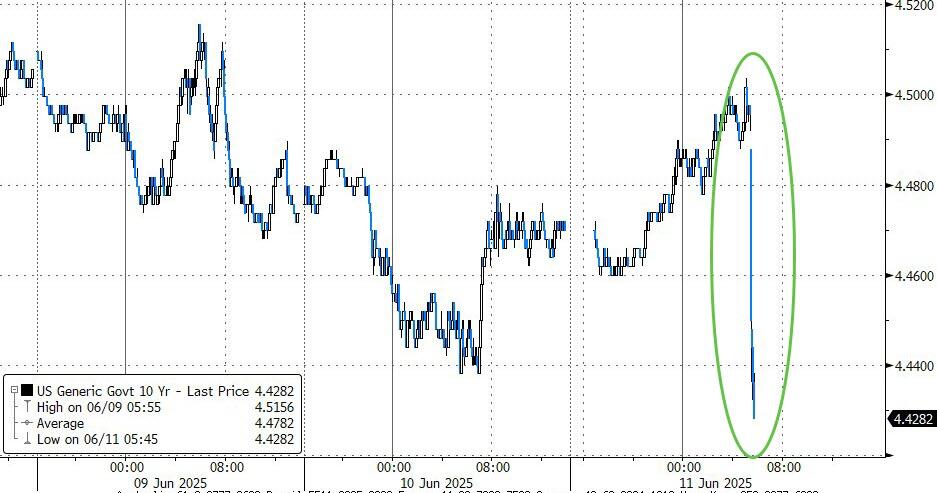
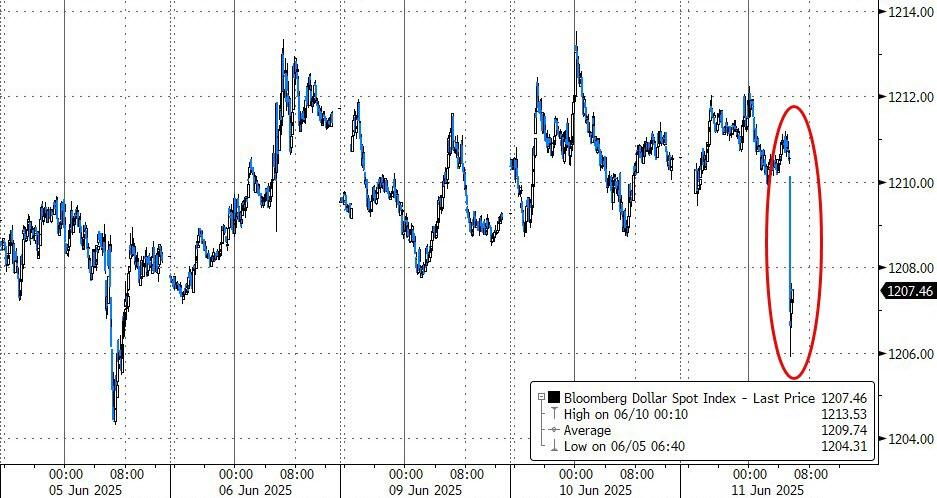
The UK pharma industry has reacted angrily to the news that the government has doubled the rate that drugmakers must repay on the sales of newer products to the NHS under the so-called statutory scheme.
The Association of the British Pharmaceutical Industry (ABPI) said this morning that the government will hike the rate to 31.3% in the latter half of this year from 15.5% in the first half, despite its warnings that the move would threaten growth and investment in the UK life sciences industry.
The scheme sits alongside the Voluntary Scheme for Branded Medicines Pricing, Access and Growth (VPAG) in setting a yearly cap on the total allowed sales value of branded medicines to the NHS each year, with sales above the threshold paid back to the government via rebates.
The rate for the latter half of the year brings the full-year average to 23.4% – rising to 24.3% next year and 26% in 2027 – which the ABPI pointed out is "well over three times what is required in Germany […] and four times the average payment rate in France."
It also said that the UK now invests a smaller share of its overall healthcare budget on medicines than any comparable country, at just 9% compared to 17% in Germany and Italy, and 15% in France.
Most companies opt for the voluntary scheme, although, a massive increase in the rebate in the last few years prompted some to opt out and switch to the statutory scheme in protest.
The decision to press ahead with the steep increase comes after a consultation period with the industry, which appears to have ignored concerns raised, and despite an acknowledgement that the voluntary scheme needs to be reviewed to avert negative impacts on industry investment and the possibility that the UK may miss out on new product launches, according to the ABPI.
It has already been speculated that AstraZeneca's termination of a plan to invest £450 million in a new vaccine manufacturing plant in the UK came about in part because of the big increase in the VPAG rate for newer medicines from around 15% in 2024 and mid-single digits in the pre-pandemic era.
"The UK's sky-high and unpredictable payment rates send a terrible message to international investors at a time when the UK is trying to position life sciences research and development as an engine for health and growth," said ABPI chief executive Richard Torbett.
"We urge the government to chart a clear path to reverse the UK's historic underinvestment in medicines, through meaningful changes to both the voluntary scheme and statutory scheme," he added.
Research (PDF) commissioned by the trade organisation has claimed that, if rates stay above 20%, the UK could lose out on £11 billion ($14.8 billion) of R&D investment by 2033, but should they be maintained below 10%, that could increase GDP by £61 billion over the next 30 years.
https://pharmaphorum.com/news/uk-follows-through-doubling-statutory-scheme-rate