By Peter A. McCullough, MD, MPH
The US Centers for Disease Control placed a public health advisory on Pfizer’s flagship oral COVID-19 drug, Paxlovid on May 24, 2022 based a a few case reports of viral rebound.
A new prospective randomized trial by Yang and colleagues confirmed shockingly high rates of virologic 21.1% and symptomatic 24.5% rebound at 28 days. In this study Paxlovid was found to be no better than mindeudesivir (VV116) which is essentially an oral form of remdesivir.
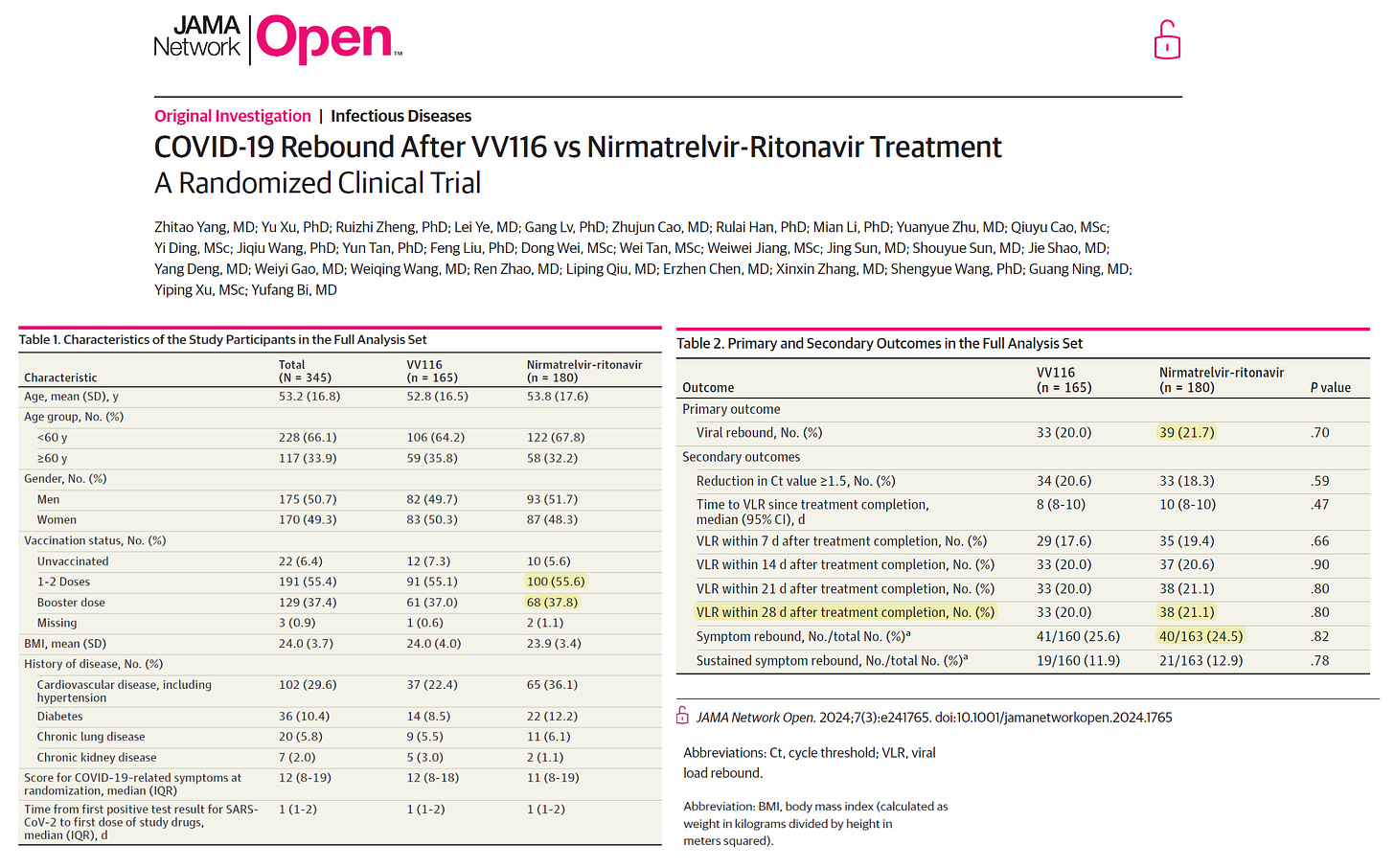
These data have important implications: 1) rebound with Paxlovid is unacceptably high at 28 days and is prolonging the infection, worsening the duration of spread, 2) mindeudesivir (VV116) has the same problem. It appears patients are much better off with the McCullough Protocol using a choice of drugs (other than Paxlovid) in combination to resolve the syndrome rapidly without risk of rebound.
https://petermcculloughmd.substack.com/p/paxlovid-rebound-proven-in-prospective
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.