The US FDA is under tremendous political pressure to approve drugs, and indeed they tout the number of approved drugs as a measure of the quality of their work. Unfortunately, you can approve bad drugs.
Selenexor is a toxic poison that does not increase survival.
Belantumab causes eye damage and had to be pulled from the US market
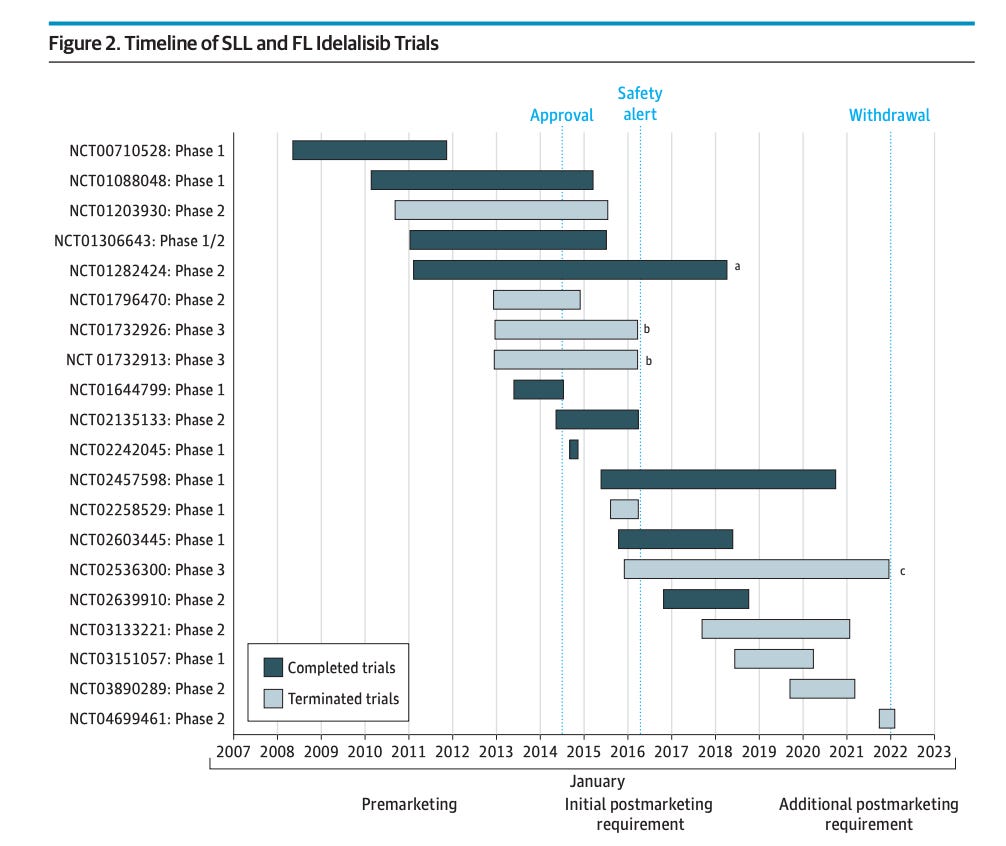
Moreover the FDA knew this for years without pulling the products. Note the time from safety alert to withdrawal, and when the data was generated.
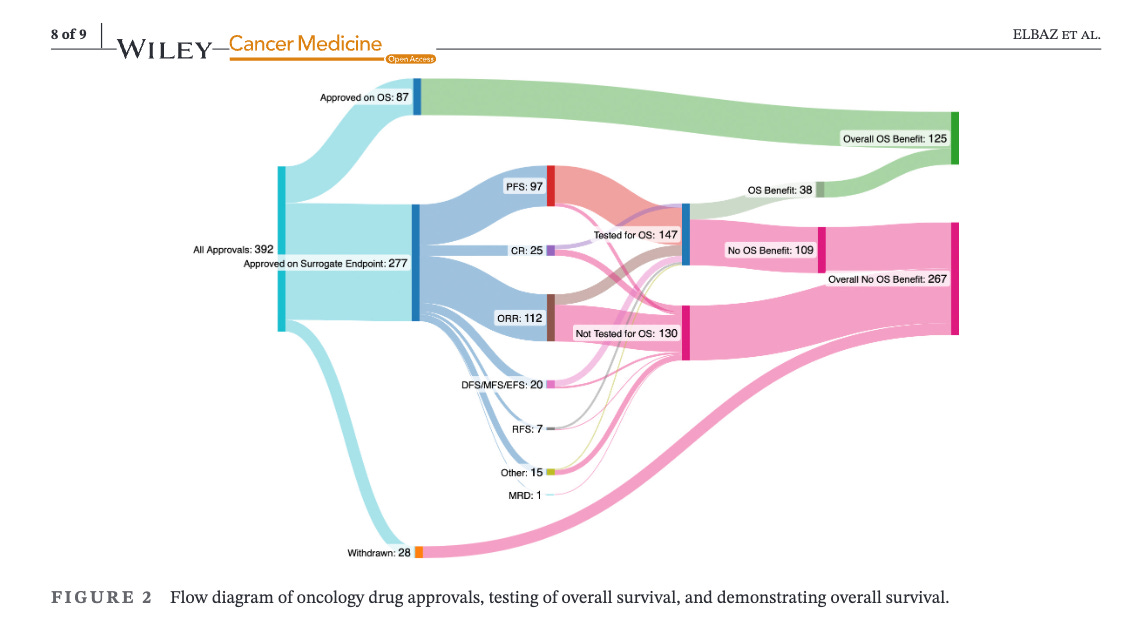
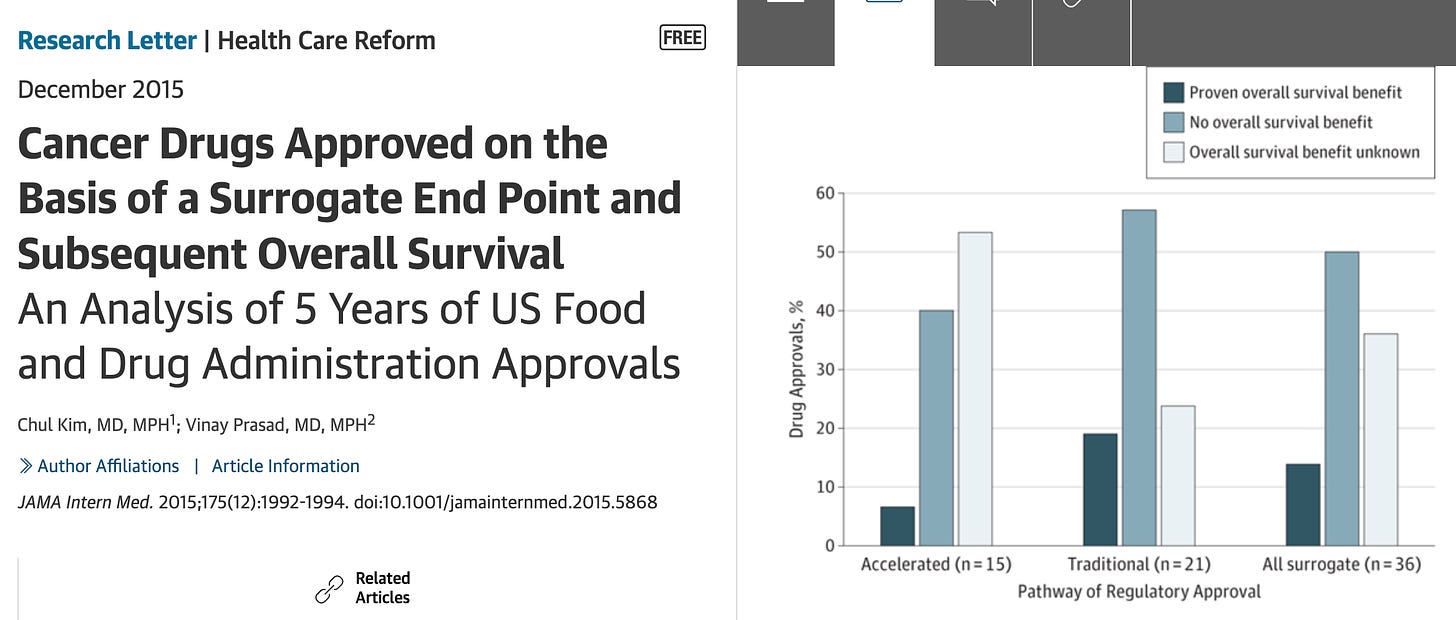
In the latest project from our team , led by medical student Josh Elbaz, we examine how many FDA approvals from 2006 to 2023 improve survival. Free link here. Here is the key figure (look at the green)
If you look at 392 approvals, just 87 (22)% are initially granted because the drug improves survival. If you don’t improve survival when you are approved, only 38 of 277 (13.7%) later do.
In other words, if the FDA doesn’t ask for survival benefits at the outset, they seldom get survival benefits.
28 drugs were withdrawn, but hundreds more remain on the market.
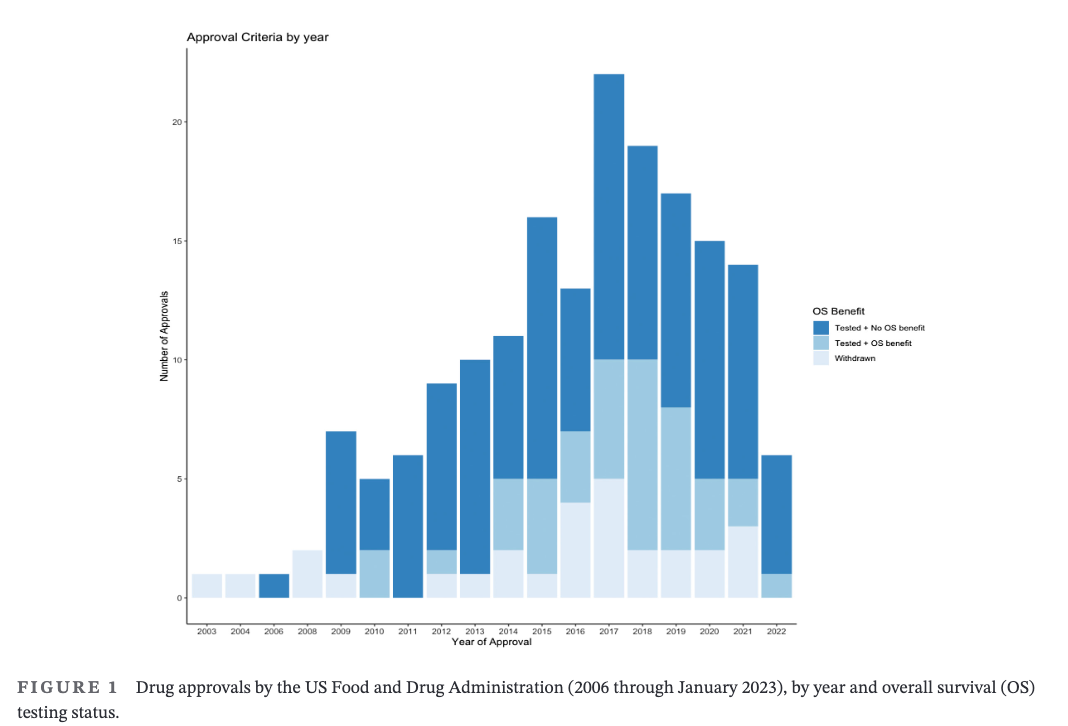
The second big finding is that this isn’t because companies don’t have enough time.
Even the older approvals consist of many drugs without survival benefits.
What gives?
Some argue that it is unrealistic to ask cancer drugs to improve survival
That argument doesn’t make sense when you consider how lethal cancer is. People with relapsed cancer are dying. You could easily run trials assesing OS benefit. Our team has shown that this will not even take more time than establishing response rates and duration of response.
A trial powered for survival in penta-refractory patients can result in less than 2 years.
People with pancreas cancer are dying. You don’t need to accept a surrogate endpoint in pancreas cancer because survival, sadly, is so short.
In some disease states, survival is long (newly diagnosed breast cancer), but here the FDA could ask for a trial to show survival benefit in refractory or late stage breast cancer, but they often do not.
Moreover, even if survival is long, with large sample size you can find survival gains fast. Jupiter is a statin trial. It found a survival benefit in 2.3 years with over 90% surviving in both arms.
In lung cancer, survival is poor, and yet drugs like sotorasib remain on the market without proving they increase survival and having failed post market commitments.
Why does the FDA grant regular approval if only surrogates are improved?
Regular approval means that there are no post marketing efficacy commitments, and the surrogate has to have a strong correlation with survival. The US FDA has clear guidelines for using surrogates via the regular approval pathway. But in work that Chul Kim and I did in 2016 we found this was not the case.
The FDA repeatedly violated their stated standards, and granted full approval for wobbly surrogates.
Thanks to JAMA IM editor Rita Redberg
In 2015, Rita Redberg— then JAMA IM editor took 2 papers from our group. One examined this question.
And the other examined surrogate correlations in oncology.
In the subsequent 9 years, several teams have used these methods and applied them to accelerated approvals (a subset of surrogate approvals) or non-cancer drugs, leading to a dozen papers. The best validation of your method is for others to accept it, and run with it!
Closing thoughts
The US FDA is a dangerous agency. They are approving COVID19 boosters for toddlers without any data that it helps them. COVID19 vaccines have never shown benefit with respect to clinical outcomes among people who already had COVID. Both of these approvals could have had randomized data had FDA asked for it. Alzheimers drug’s that don’t work are approved. Toxic drugs are approved for women with post-partum depression. Rare disease drugs that are ineffective remain on market, and cancer drugs are a total disaster. Most —68%!!— have no proof they extend survival.
America spends 20% of GDP on healthcare, and we are spending billions on drugs without knowing they help. There are many other worthy priorities we don’t spend on, and we are taking money from working people to transfer it to shareholders of these companies. That’s wrong.
The FDA is playing a dangerous game with Americans’ health. One day, they will approve a drug with harms that far outweigh benefits and they will not be able to conceal that story. It already happened with Pi3k inhibitors and COVID19 vaccination in young men (myocarditis harm » benefit), but the harm signal can be obfuscated with rhetoric. But that won’t last forever.
When the FDA eventually approves a drug whose harms it cannot downplay or dismiss, serious reform of the FDA will be considered. Sadly, Americans will have to pay the price until then.
Vinay Prasad Hematology Oncology Medicine Health Policy Epidemiology Professor
https://www.sensible-med.com/p/the-us-fdas-cancer-drug-approval


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.