by Vinay Prasad
Recently, some are reporting that the NIH will work to deprioritize grants that seek to utilize mRNA vaccine technology in therapeutic applications. I think this is a wise decision for 5 reasons
The brand is damaged. One of the points Jay Bhattacharya made in his confirmation hearing was about the use of medical products derived from aborted embryos. Jay said that there is a large group of Americans who will not accept these product which is a reason to reduce its funding. Whether we like it or not— acceptability among people is a factor in funding. Due to the disastrous Biden vaccine mandates and obfuscation around myocarditis and other safety signals, I fear the brand is damaged for most uses.
There are alternatives. MRNA vaccines utilize the cell to create the protein against which immunogenicity is developed. There have been and there are alternative means to do this. In the case of the covid-19 pandemic, it was extremely convenient to use mRNA because by taking advantage of the cells intrinsic ability to create proteins we could go very quickly, which was at a premium because of lockdowns and closure (our bad policy). In other therapeutic arenas, that speed does not have the same salience.
The long term safety profile is unknown. While this does not apply to uses in populations facing imminent death, it does apply to other infectious uses or in diseases with long survival. I tend to be skeptical of claims of unknown side effects until they are shown with validity. At the same time I know that the current safety program is extremely poor at characterizing safety signals. We do not yet know the long term impact of this technology, and again, if you have a terminal disease, the risks of long-term safety concerns are less important.
Uses in cancer are of low pretest probability. With colleagues, we have a paper forth coming looking at the randomized control trials of cancer therapeutic vaccines. Spoiler alert: it is a sea of negative findings. What precisely is a cancer therapeutic vaccine? We take patients who have cancer, whose immune system maybe impaired or senescent, and we inject them with antigens against their tumor. Those antigens can be made with protein derivatives, or they can be given as an MRNA. But the idea is the same to sensitize the body against antigens in the body. These can— and have been— even been bespoke— made for each individual. All of these trials have failed. Provenge came to the US market but the trial has misuse of crossover, so the difference is likely due to docetaxel administration.
From our paper (2015 above)
The pretest probability for cancer therapeutic vaccines is lower than other domains of cancer biology, such as cytotoxic drugs, tyrosine kinase inhibitors, antibody drug conjugates and even cellular therapy. That is not to say that the pretest probability is zero. It never is, and something may succeed against the odds, but the role of funding is to try to improve one’s chances.
If this class of product is so transformational and will be so well received by the American people then surely the companies that have already invested billions of dollars, and have hundreds of billions of dollars in cash would invest further in these products. But they are also very interested in these federal subsidies.
Side note: Some cite these two papers to support mRNA vaccines
This is a phase 1 study without a control arm.
They took pancreas cancer patients with a single resectable panc lession. Did surgery. Had to have path R0 or R1 resection! Gave atezo, gave their bullshit vaccine, then gave FOLFIRNOX.
They present an analysis by biomarker plot in the paper.
No control arm. Some people destined to well already. Restricted to R0 or R1 resection (aka cherry picking) and the intervention was confounded with FOLFIRINOX.
A completely useless study.
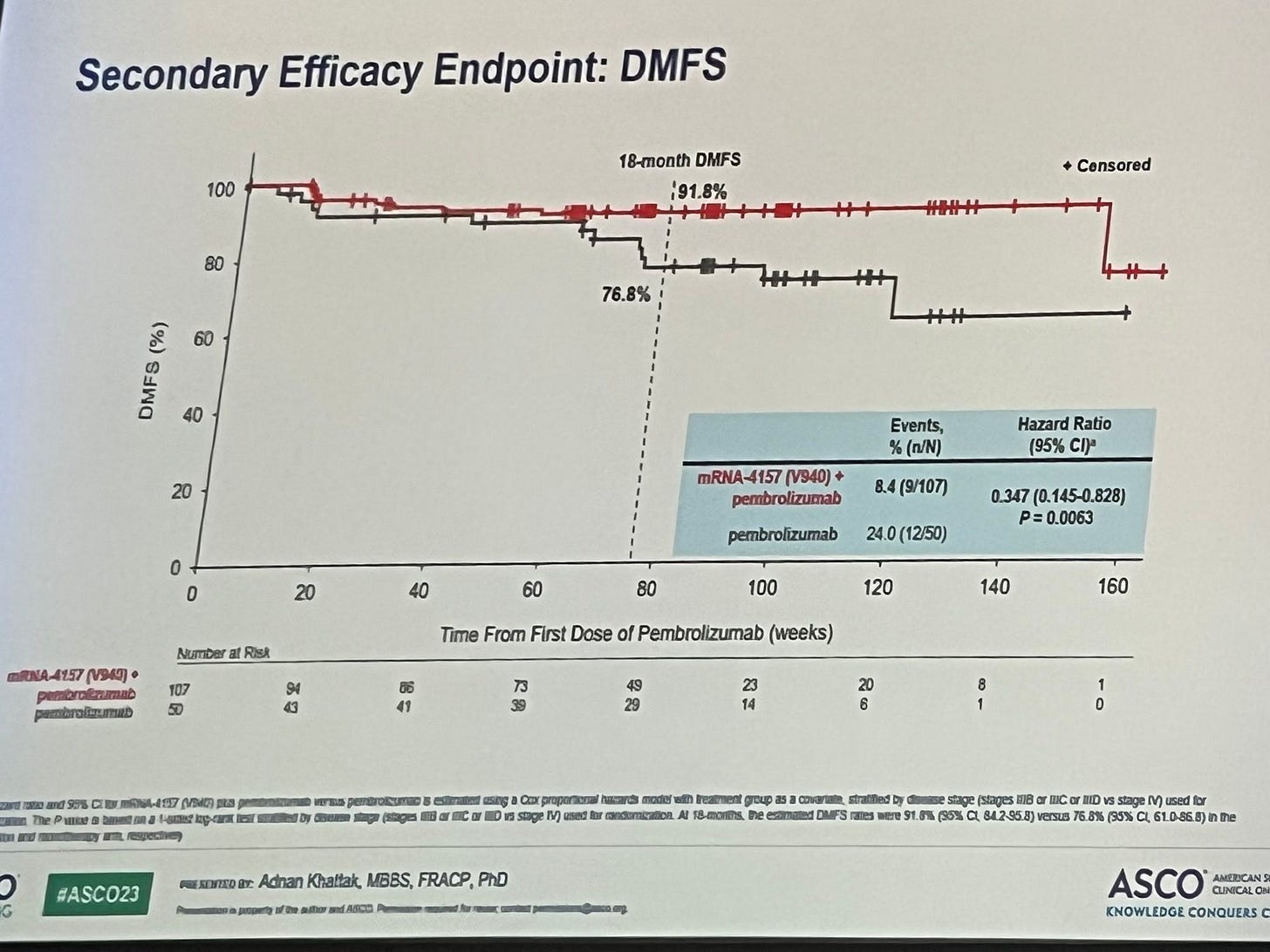
At least this is randomized
Problems. Volatile estimates, tons of censored patients, cherry picked 18 month time point.
If this melanoma vaccine is so good, what is it’s response rate in relapsed refractory metastatic disease?
I will bet it fails in large phase 3 without high drop out.
https://www.drvinayprasad.com/p/yes-mrna-vaccine-science-should-be



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.