About 17 people die each day in the US waiting for an organ transplant — 6200 per year. And for the more than 10,000 Americans on the waiting list for a liver transplant, the future of medicine may lie not in a donor list, but in a ghost.
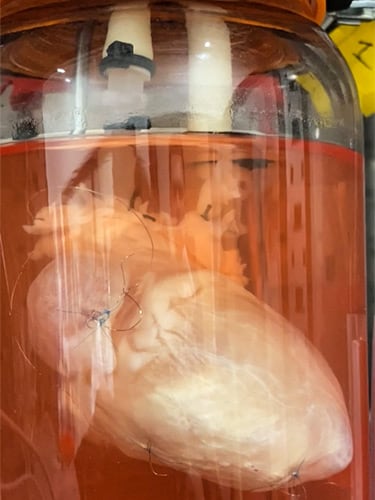
The term “ghost organ” refers to a bioengineering process where an existing organ is gently stripped of all its native cells. What remains is a pristine, translucent protein scaffold — the extracellular matrix (ECM) — nature’s architectural blueprint for the organ. The scaffold is then recellularized, seeded with either new cells from the patient, healthy donor cells, or patient stem cells — a method that theoretically can be used to create any organ or tissue in the human body.
For years, the goal of decellularization/recellularization technology to grow a rejection-proof organ on demand felt like distant science fiction. But recent breakthroughs have made that fiction a reality.
This past June, Miromatrix Medical Inc., a subsidiary of United Therapeutics, announced a win in the first clinical trial with its manufactured organ alternative: the miroliverELAP®. The miroliverELAP® system, an external liver assist device, consists of a decellularized pig liver scaffold that is repopulated with donated human liver cells, creating a bioengineered liver that functions outside the body to provide temporary support.
This external liver system is the first of its kind to enter human clinical trials and is connected through an external blood circuit to replicate the essential functions of a healthy liver.

The successful procedure was performed at Intermountain Medical Center in Murray, Utah, led by Christopher J. Danford, MD, a transplant hepatologist at Intermountain Health. The patient who received the liver was ineligible for a liver transplant and experiencing liver failure.
The external liver, placed in a box, was perfused for 48 hours in a similar fashion to dialysis, according to Danford, with the aim of testing safety and efficacy. “It was very nerve wracking. [I was thinking] would this clot off? Would it cause unforeseen problems? It was exciting, but I also did not get much sleep,” Danford said.
Fortunately, no unforeseen problems arose, and the organ successfully performed the critical functions of a healthy liver, providing one of the most tangible signs yet that lab-grown, transplantable solid human organs could be a real solution.
Miromatrix’s phase 1 trial is still open for enrollment across eight US sites. Though the trial was initially opened only for people with acute liver failure, it has since been expanded to include acute liver failure and alcoholic hepatitis.
How De-Cell/Re-Cell Process Works
The core idea behind de-cell/re-cell technology to build a new organ is to strip the ECM of its original cells and then repopulate that natural scaffold with new cells.
Miromatrix’s own technological platform is based on using decellularized porcine (pig) organs. Their proprietary process uses perfusion decellularization and recellularization, in which all the pig cells are gently removed from the organ while leaving the intricate protein structure, or scaffold, perfectly intact.
In essence, a ghost organ is the underlying framework of an organ. Scientists perfuse the organ with a series of mild detergents and enzymes that act like soap, washing away all the cellular material — including DNA and anything that could trigger an immune rejection.

“We use the blood supply of that organ to put a solution through, and because the blood supply reaches every cell, it lyses every cell in the organ, and then you can wash all that cellular goo out,” said Doris A. Taylor, PhD, CEO of Organamet Bio Inc. Taylor, a cofounder of Miromatrix no longer affiliated with the company, now focuses on cardiovascular regenerative medicine, refining the de-cell/re-cell process for whole hearts with Organamet Bio.
At Organamet, once the heart cells are lysed out and the heart scaffold is created, they’re seeded with the patient’s own induced pluripotent stem cells. These stem cells can differentiate into any human cell type, such as hepatocytes in the liver, smooth muscle cells in arteries and veins, and cardiomyocytes in the heart. Because they’re from the receiving patient, they create a personalized organ that won’t be rejected by the recipient’s immune system, potentially eliminating the need for lifelong immunosuppressant drugs.
Once the cells are seeded, nature takes over with the right nudge from the engineers fashioning the organ.
“The matrix teaches the cells what to do if we rebuild the vascular tree first and then add a mixture of the appropriate cardiac mesoderm cells and put them in throughout the heart. We then give them the right mechanical and electrical cues,” said Taylor.
The last step is the maturation process. Advanced bioreactors mimic the human body to mature these reseeded hearts into strong, functional organs capable of supporting life.
Possibly the most challenging aspect in building any functional solid organ is relining the organ’s entire vascular “plumbing” with a new layer of endothelial cells so they can mature and handle the full workload of living tissue as it involves solving two monumental problems simultaneously: preventing immediate, catastrophic blood clotting, and re-establishing the complex, living functions of the vascular system on a massive scale.
Endothelial cells lining the vasculature sense blood flow and release molecules that regulate blood pressure and inflammation. Shear stress due to blood flow could strip away a weak lining, and edema may result if it’s too leaky.
The ECM scaffold — now exposed after cells are washed away — is rich in proteins like collagen, but highly thrombogenic. It instantly signals blood platelets to stick. Preventing thrombosis is critical to avoid blocking major vessels, which would starve the organ of oxygen and cause it to die within minutes to hours of transplantation.
“If you can’t get a blood supply to cells, they die,” said Taylor of the importance of the vasculature. “The cells in the middle die because they don’t have a blood supply. The cool thing about these decellularized scaffolds is the blood vessel pathway is still there, and that’s what makes it unique.”
On the success of Miromatrix’s liver device, Taylor said, “I was very excited. This opens the door to saving lives and it begins to give patients hope, it gives clinicians data, and it gives the world a perception that this is no longer science fiction, it’s becoming medicine. It’s the culmination of what we started in my laboratory 20 years ago. I couldn’t be prouder to see it come to fruition.”
Engineered Blood Vessels On-Demand
While the dream of transplanting solid organs like hearts and livers grown from decellularized scaffolds remains on the horizon, one company has brought the technology to life by focusing on connective tissue: blood vessels.

Laura Niklason, MD, PhD, founder and CEO of Humacyte, has helped translate de-cell/re-cell tech into a solution for those with traumatic injury affecting their circulatory system, creating acellular tissue engineered vessels.
The vessels are created by culturing human vascular cells on a biodegradable, tube-shaped scaffold in a bioreactor bag designed to provide the natural mechanical stimulation to ensure the vessel is viable. After the cells produce a robust, natural matrix, they are gently washed away, leaving a resilient, off-the-shelf “ghost” vessel that is universally implantable and resistant to infection.
“We grow the vessels in bioreactor bags. Each bag contains one vessel. The vessel is 42 cm long and 6 mm in diameter…we then decellularize it in the bag and then ship it and store it in the hospital. After that, we never open the bag until it gets opened by the surgeon,” said Niklason, also the Nicholas M. Greene Professor of Anesthesia and Biomedical Engineering at Yale University, New Haven, Connecticut.
The vessels are now FDA approved to treat traumatic injury, such as for patients who have had vascular injury from car accidents or gunshot wounds.
But their potential indications could extend wider, such as for those with peripheral vascular disease. Patients with blocked arteries in the legs have leg ischemia and need revascularization, which Niklason said has seen “really encouraging results [in clinical trials].” Another potential indication is patients who have kidney failure, who are on dialysis and need a conduit that goes from an artery to a vein.
To ensure the decellularization process in Humacyte’s blood vessels doesn’t damage critical ECM components, the company utilizes a panel of assays for quality control, including a test for suture retention strength and burst strength to make sure the material can withstand the continuous force of arterial blood pressure without leaking or rupturing.
On top of all that, quantitative proteomics is performed to test the matrix molecules and ensuring regenerative potential. “It’s really that signature that stimulates cells to repopulate over time,” according to Niklason.
Though Niklason has been working with this technology for decades, what keeps her moving forward is solving the challenge of turning what is essentially an analog science — biology — into a digital science like computing. “By really studying the problem of how to grow an artery over the last couple decades, we have figured out how to get this analog biology to behave the same way every time. So we’ve almost given it a digital level of control,” she said.
As for the first successful transplant of a lab-grown solid organ, like what Organamet Bio is working to do with the heart, that could be much closer than we think. “There is major work underway with lung, heart, liver, and kidney,” said Taylor. “Right now, we have the technology to get to first in-human with heart within 5 years, if we have the right amount of funding. It’s all about funding.”
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.