Activist investor Carl Icahn, founder of Icahn Enterprises (IEP), has built a large stake in Cigna (CI) and intends to vote against the insurer’s $54B proposed acquisition of Express Scripts (ESRX), the Wall Street Journal reports, citing people familiar with the matter. Icahn, whose stake amounts to less than 5% of the company’s outstanding shares, believes Cigna is paying too high a price for Express Scripts, the report says. Icahn is leaning toward attempting to convince other investors to oppose the deal as well, the report notes. Shares of Express Scripts are down 6.3% after the news.
Search This Blog
Wednesday, August 1, 2018
Migraine Prevention: Botox Wins, but Another Drug Enters the Ring
Hello. I’m Dr Arefa Cassoobhoy, a practicing internist, Medscape advisor, and senior medical director for WebMD. Welcome to Medscape Morning Report, our 1-minute news story for primary care.
For decades, the prophylactic options for migraine were limited, and a number of migraine sufferers either didn’t respond to or didn’t tolerate the available drugs.
In 2010, onabotulinumtoxin A (Botox®) injections were approved for the prevention of chronic migraine. But since then, in meta-analyses,[1] Botox has had only a small to modest benefit over placebo.
The biggest advantage to Botox might simply be its acceptance by patients. In a recent open-label trial funded by Allergan and presented at the American Headache Society meeting, Botox was compared with topiramate, a first-line migraine prevention drug.
Of those patients completing the trial, 40% in the Botox arm achieved a 50% reduction in headache days compared with 12% in the topiramate arm. What’s striking is that more than 80% of the patients stopped topiramate because of intolerable side effects or lack of efficacy, compared with 18% of the Botox group. However you define “success” in migraine prevention, the patient must be able to tolerate the treatment.
The newest option for the prevention of chronic migraine is Aimovig™, the first monoclonal antibody. It’s a calcitonin gene–related peptide inhibitor that targets the inflammation that’s believed to cause migraine. We’re waiting to see whether Aimovig will be viewed as a second- or third-line preventive therapy. Will patients be required to fail not only traditional migraine drugs, but also Botox, before they can try it?
Aimovig has the advantage of being a monthly self-administered injection; however, it’s expected to be more costly. And, at this stage, long-term safety data are limited. But with three other drugs of this class in the pipeline, clinicians will need to become familiar with these drugs to guide their patients’ treatment.
Theranos Lied, but These Companies Really Are Revolutionizing Blood Tests
If Theranos’ promise of a comprehensive blood test from a drop of blood sounded too good to be true, that’s because it was. The $9 billion startup, led by CEO Elizabeth Holmes, promised a healthcare revolution and the commercialization of blood tests. When Holmes realized that her vision of a microneedle patch to take blood was not within the realm of reality, she turned to fabricating blood test results to the point of risking patient lives. Bad Blood: Secrets and Lies in a Silicon Valley Startup, by Wall Street Journal investigative journalist John Carreyrou, recounts the story of how Holmes was able to deceive Silicon Valley investors for more than a decade.[1] Carreyrou describes the Orwellian nature of Theranos and Holmes’s obsession with success, as seen through a determination for financial gain that stemmed back to her youth and later emulation of Steve Jobs.
Holmes’s promise of having a blood test reader in every home may not come to fruition any time soon, but many companies are innovating in the field of blood tests with products that have evidence to back them up. While many of these startups are facing skepticism from investors and consumers due to the infamous legacy left by Theranos, here are 10 companies that are working to repair the reputation of blood test biotechnology.
Athelas


Athelas is a finger-prick blood test that monitors platelets, white blood cells, neutrophils, lymphocytes, and more. After pricking your finger, blood is placed on a test strip which is then inserted into the Athelas device. Within a minute, you can view blood count data on your phone.[2] Athelas is primarily targeted to oncologists, who can monitor their patients’ immune systems while they’re undergoing chemotherapy from the comfort of their homes. Athelas is currently awaiting approval by the US Food and Drug Administration (FDA) and provides all accuracy data and research methodologies to the public. The startup was founded in 2016 by CEO Tanay Tandon when he was just 17 years old, and has already managed to raise $3.7 million.
Cholestech
Abbott’s Cholestech LDX Analyzer is a device that tests blood glucose and cholesterol levels. Cholestech is able to test total cholesterol as well as low-density and high-density lipoprotein cholesterol levels.[3] Through Cholestech, physicians can monitor their patient’s disease risk or treatment effect for diabetes, heart disease, and other cardiovascular diseases. Abbot states that Cholestech provides “immediate awareness and identification of cardiovascular and diabetes health risks in only 5 minutes.”[4]
Grail Inc.
Since 2016, Grail Inc. has raised $1.5 billion towards its liquid biopsy products.[5] Through genetic sequencing, Grail aims to detect cancers before the emergence of any symptoms. The method is known as “high-intensity” genomic sequencing, which picks out DNA released by tumors into the blood. A recent clinical trial of Grail, including patients from Mayo Clinic and Memorial Sloan Kettering, found that Grail correctly recognized liver and ovarian cancers 80% of the time, with lower accuracies for breast cancer (detected 21% of the time), prostate cancer, and melanoma (both of which had under 10% accuracies).[6]
Genalyte

Genalyte’s Merlin 1.0 device is a portable trolley that can perform blood tests, typically sent to laboratories, in the convenience of a doctor’s office or surgical theater. Genalyte also claims that its device can provide results in 15 minutes rather than the standard few days that labs require, and can run up to 128 different tests through a single drop of blood.[7] CEO Cary Gunn recognizes that Genalyte seems to be heir to the throne, or void rather, left behind by Theranos, but asserts that rather than trying to reinvent the wheel, Genalyte is simply bringing the blood tests already conducted in labs right to the doctor’s office.[8]
Orphidia
Orphidia aims to transform the healthcare industry through single-use disposable chips for lab testing. Results of 40 different blood tests performed on a small sample of blood can be sent from a portable tabletop analyzer to your phone in 20 minutes.[9] The Orphidia analyzer can monitor various biomarkers in the blood and send data to any device through a secure cloud. Founded in 2013, Orphidia has raised over $3 million in seed funding.[10]
Karius


The Karius test is a next-generation sequencing assay to identify pathogens for diagnosing infectious diseases. The test can identify more than 1000 pathogens, including more than 750 bacteria, 300 molds and fungi, and 100 DNA viruses.[11] In order to run the Karius test, a standard 5-mL whole blood test vial is required. Results are delivered as a one-page report, outlining pathogens that were prevalent at significant levels.
Abaxis
Founded in 1989, Abaxis is not new to the biotechnology startup scene. Abaxis has developed a method of manufacturing stable reagents in extremely small quantities, known as Orbos technology.[12] Orbos technology is used to measure various parameters from minute amounts of blood, which led to the development of the Piccolo analyzer in 1995. The Piccolo analyzer uses reagent discs to complete 31 on-site diagnostic tests, requiring about three to four drops of blood. In the same year, the VetScan analyzer was released to meet the same needs for veterinarians.
Now Diagnostics

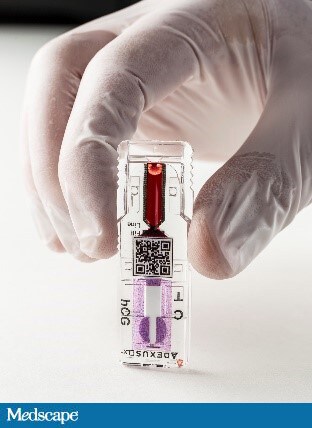
Now Diagnostics is similar to the other startups mentioned here in that it is able to complete a blood test in minutes with just one drop of blood. Rather than one device that can complete a plethora of tests, however, Now Diagnostics’ product line caters to specific conditions.[13] Its ADEXUSDx line includes tests for pregnancy, heart attacks, toxicology, sexually transmitted diseases, food allergies, and common infectious diseases. Diagnostic tests range from detecting syphilis, to strep throat, to a shellfish allergy. Now Diagnostics states that its pregnancy test, First To Know, is 99% accurate and can provide results within 10 minutes.[14]
Day Zero Diagnostics
Day Zero Diagnostics also employs genomic sequencing in its technology but uses it to identify antibiotic resistance. Day Zero states that its diagnostic test can identify the species and antibiotic resistance of a pathogen within hours, as opposed to the 2-5 days required by a lab.[15] Through this assessment, physicians can use targeted antibiotics to treat their patients, reducing the length of hospital stays, cutting costs, and avoiding the 8% increase in mortality per hour associated with the typical time delay for blood test results. Day Zero won the 2017 MedTech Innovator Global Competition, receiving $350,000.[16]
rHEALTH
Only one drop of blood is required for the rHEALTH reader, which can identify over 100 diseases and communicate to your smartphone via the SKYE sensor and Bluetooth.[17] rHEALTH is also currently partnered with NASA and says on its site that even an astronaut crew member can use its device for a self-diagnosis without any technical training. Beginning in 1998 as a software company, rHEALTH shifted its focus to the healthcare field in 2006 and aims to decentralize laboratory blood tests through wearable devices.[18]
Pluristem, Rexahn Biotech Winners, B. Riley Says In Bullish Initiation
With hundreds of biotech stocks all assuring big prospects, building a portfolio of truly transformative medicine makers is a feat. B. Riley FBR said it’s found two winners.
The Analyst
Analyst George Zavoico initiated coverage of Pluristem Therapeutics Inc. PSTI 0.8% and Rexahn Pharmaceuticals, Inc. RNN 0.33%with Buy ratings and respective price targets of $4.50 and $7.50.
The Pluristem Thesis
B. Riley’s Pluristem bullishness is predicated on “a unique regenerative medicine platform technology, a wholly owned state-of-the-art manufacturing facility and two differentiated cell therapy product candidates in clinical development for five indications,” Zavoico said in the initiation note. (See the analyst’s track record here.)
The candidates, PLX-PAD cells, target conditions for which there are no “satisfactory” treatment alternatives. B. Riley has high hopes.
“In our view, the results of preclinical studies and earlier-stage clinical trials provide evidence of efficacy and safety,” Zavoico said. “If confirmed in later-stage trials, these products could become the next standards of care.”
The near-term completion of two Phase 3 PLX-PAD trials, the potential filing of Biologics License Applications in 2020 and 2021 and the potential fourth-quarter launch of a pivotal trial for PLX-R18 could drive additional value, the analyst said.
The Rexahn Thesis
Zavoico considers Rexhan’s oncological platform — including RX-3117 for metastatic pancreatic cancer and supinoxin for triple negative breast cancer — differentiated from standard alternatives with “unsatisfactory” safety and efficacy profiles.
“With positive preclinical and early clinical proof-of-concept results showing RX-3117 and supinoxin to be well-tolerated with early evidence of efficacy, we view these drug candidates as having the potential to provide patients with a superior treatment option for difficult-to-treat cancers,” the analyst said.
B. Riley forecast a commercial launch of RX-3117 in 2022 in a combination therapy with Abraxane. Supinoxin is seen to follow in 2024 in combination with a checkpoint inhibitor. The two drugs are in Phase 2a trials.
To fund their advancements, Rexhan is expected to raise capital in the fourth quarter after presenting interim RX-3117 data and top-line supinoxin data, Zavoico said.
Cytokinetics Downgraded On Data Delay
Biopharma Cytokinetics Inc. CYTK 5.78% announced a delay in drug studies during its earnings call Thursday, prompting a downgrade by Morgan Stanley.
The Analysts
Morgan Stanley’s Matthew Harrison downgraded Cytokinetics from Overweight to Equal-Weight and lowered the price target from $18 to $9.
The Thesis
Morgan Stanley’s valuation for Cytokinetics is partly driven by the base value in omecamtiv mecarbil, the company’s cardiac myosin activator, which is being studied for heart failure, Harrison said. (See the analyst’s track record here.)
Key data for this treatment is unlikely for a few years.
Cytokinetics additionally announced that data for reldesemtiv in the large ALS study will be delayed, with data expected in early 2019.
This delay is due to the patient availability and uptake of Radicava, an ALS treatment, according to the biotech.
The main catalysts for Cytokinetics are near-term, and the stock is ikely to trade evenly with the market until advancements are nearer, Harrison said.
In the midst of the delays, Galactic-HF, an omecamtiv mecarbil program in heart failure, is on track with over 50-percent enrollment and 4,000 patients, according to Morgan Stanley.
“We remain positive on the Amgen, Inc. AMGN 0.48%-partnered program and believe the study is likely to succeed,” Harrison said.
Morgan Stanley Buys Sarepta Sell-Off, ‘$600M Recurring Opportunity In DMD’
A manufacturing-related hold on Sarepta Therapeutics Inc SRPT 5.99%’s Duchenne muscular dystrophy candidate catalyzed a recent sell-off. Morgan Stanley sees a buying opportunity in the otherwise-lauded program’s stumble.
The Analyst
Analyst Matthew Harrison upgraded Sarepta from Equal-weight to Overweight and maintained their $163 price target.
The Thesis
Sarepta has fallen 25 percent off its mid-June, post-data high, but Morgan Stanley is dismissing the pullback.
“We believe the decline has occurred as shareholders transition from mostly short-term to more longer-term oriented investors,” Harrison said in a Wednesday note. (See the analyst’s track record here.)
“Importantly, we do not believe a major bear case has emerged on the prospects for their DMD gene-therapy program.”
A hold on Sarepta’s Phase 1/2 trial is expected to be resolved without delaying a pivotal trial planned to start by the end of the year. Morgan Stanley anticipates drug approval in 2021, but notes that the Food and Drug Administration could accelerate the push to market.
Sarepta’s candidate is seen to lead rivals from Solid Biosciences Inc SLDB 7.19% and Pfizer Inc. PFE 0.58% in both efficacy and timing. Its $600-million recurring market opportunity, which presents a revenue stream that could double Sarepta’s market cap, is not reflected in its valuation, Harrison said.
“While competition and manufacturing could present headwinds, we believe management will solve any manufacturing hurdles quickly and believe management’s lead on competition significantly reduces the competitive threat,” the analyst said.
New data for the recently acquired limb girdle muscular dystrophy program could also drive upside, according to Morgan Stanley.
August FDA dates
Biotech stocks had a fairly decent run up in July after a nearly flat performance in June. The iShares NASDAQ Biotechnology Index (ETF) IBB 0.51% is up about 5 percent.
As the earnings season kicks into high gear and amid several other catalysts, August is likely to be an action-packed month. Here are some key PDUFA action dates a biotech investor should stay focused on.
Pain Therapeutics Looks For Alleviation Of Pain Inflicted By Negative FDA Panel Vote
- Company: DURECT Corporation DRRX 0.68% and Pain Therapeutics, Inc. PTIE 10.27%
- Type of Application: NDA
- Candidate: Remoxy
- Indication: severe pain
- Date: August 7
Pain Therapeutics has licensed Remoxy, an abuse-deterrent extended release capsule formulation of oxycodone, from DURECT. Pain said in mid-February it resubmitted the NDA for FDA review. The previous application was issued a complete response letter in Sept. 2016. Remoxy is indicated to treat severe pain.
Meanwhile, in an adverse development, a FDA panel that met in late June to discuss the NDA voted 14 to 3 against approval of Remoxy for managing pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate.
The stock, which shed about 71 percent following the FDA panel vote, hasn’t recovered.
Alnylam Awaits Approval Of Drug To Treat Hereditary ATTR Amyloidosis
- Company: Alnylam Pharmaceuticals, Inc. ALNY 0.62%
- Type of Application: NDA
- Candidate: Patisiran
- Indication: Hereditary ATTR, or hATTR, amyloidosis
- Date: August 11
Patisiran is an investigational RNAi therapeutic targeting transthyretin, or TTR, for treating hATTR amyloidosis. It has been accorded priority review status and also has a Breakthrough Therapy designation.
hATTR amyloidosis results from mutations in the TTR gene, which causes abnormal amyloid protein to accumulate and damage body organs and tissues such as peripheral nerves and heart.
Alnylam uses Arbutus Biopharma Corp ABUS 4.05%‘s intellectual property in RNAi therapeutic products using LNP technology.
Regeneron’s Twin FDA Review Events
- Company: Regeneron Pharmaceuticals Inc REGN 0.66%
- Type of Application: sBLA/sBLA
- Candidate: Eylea / Praluent with apheresis
- Indication: Wet Age-related Macular Degeneration, or AMD/treating elevated low-density lipoprotein cholesterol
- Date: August 11/ August 24
The FDA had accepted Regeneron’s aBLA for a 12-week dosing interval of Eylea in patients with wet AMD last December. Eylea is recommended in a 2mg dosage, administered as injection in the eye, every eight weeks following three initial monthly (four-week) injections.
Regeneron and partner Sanofi SA (ADR) SNY 1.22% are also knocking the FDA altar for using Praluent injection in patients with heterozygous familial hypercholesterolemia with apheresis, an invasive procedure to remove LDL cholesterol from the blood.
Praluent, chemically Alirocumab and a PCSK9 inhibitor, is used along with diet and maximally tolerated statin drugs to lower LDL cholesterol in blood.
Amicus’ Fabry’s Disease Drug Awaits FDA Approval
- Company: Amicus Therapeutics, Inc. FOLD 0.21%
- Type of Application: NDA
- Candidate: Migalastat
- Indication: Treating patients 16 years and older with Fabry disease who have amenable mutations
- Date: August 13
The FDA accepted the NDA for Migalastat, an oral precision medicine, according it priority review status. The candidate had previously received Orphan Drug as well as Fast Track designations. It has already been approved in the European Union.
Fabry’s disease is a type of lysosomal storage disorder and is an inherited disorder that results from the build up of a type of fat called globotriaosylceramide in body’s cells.
Bristol-Myers Seeks FDA Nod For Opdivo To Treat Small-Cell Lung Cancer
- Company: Bristol-Myers Squibb Co BMY 1.93%
- Type of Application: sBLA
- Candidate: Opdivo
- Indication: Small-cell lung cancer, or SCLC
- Date: August 16
The FDA accepted the sBLA for Opdivo, indicated to treat patients with SCLC, whose disease has progressed after two or more lines of therapy. The application was supported by safety and efficacy data from the SCLC cohort of the Phase 1/2 CheckMate-032 trial evaluating Opdivo monotherapy following platinum-based chemotherapy.
Mallinckrodt’s Infantile Jaundice Drug Up Before FDA
- Company: Mallinckrodt PLC MNK 0.72%
- Type of Application: NDA
- Candidate: Stannsoporfin
- Indication: Treating neonates at risk for developing severe hyperbilirubinemia, or severe jaundice
- Date: August 22
Mallinckrodt has added Stannsoporfin to its pipeline through its acquisition of privately-held InfaCare.
KALA Awaits FDA Clearance For Post-ocular Surgery Pain Treatment
- Company: Kala Pharmaceuticals Inc KALA 1.02%
- Type of Application: NDA
- Candidate: Inveltys
- Indication: inflammation and pain in patients who have undergone ocular surgery
- Date: August 24
Valeant’s Acne Treatment Candidate Under FDA Scanner
- Company: Valeant Pharmaceuticals Intl Inc NYSEVRX
- Type of Application: NDA
- Candidate: IDP-121 lotion
- Indication: For treating acne
- Date: August 27
Valeant’s Ortho Dermatologics unit announced FDA acceptance of the NDA for IDP-121 lotion, chemically tretinoin 0.05 percent, on Jan. 12. If approved, the company said it would be the first tretinoin product in lotion form rather than a gel or cream.
Tetraphase Seeks Approval For Antibiotic to Treat Abdominal Infections
- Company: Tetraphase Pharmaceuticals Inc TTPH 0.35%
- Type of Application: NDA
- Candidate: Eravacycline
- Indication: Complicated intra-abdominal infections
- Date: August 28
The NDA submission for Eravacycline, a fully-synthetic fluorocycline, was supported by data from the IGNITE1 and IGNITE 4 Phase 3 clinical trials.
Can Akcea’s Volanesorsen Pass The FDA Muster?
- Company: Akcea Therapeutics Inc AKCA 0.17%
- Type of Application: NDA
- Candidate: Volanesorsen, a RNAi drug
- Indication: Treating patients with familial chylomicronemia syndrome, or FCS
- Date: August 30
A FDA panel discussed the NDA of Akcea, a unit of Ionis Pharmaceuticals Inc IONS 0.18%, in May and voted 12-8 in favor of approving the drug.
FCS, a liposome disorder, is characterized by the buildup of fats in the body due to a deficiency in the enzyme lipoprotein lipase.
Can Second Time Be Charm For RedHill Biopharma?
- Company: REDHILL BIOPHAR/S ADR RDHL 2.32%
- Type of Application: NDA
- Candidate: RHB-104
- Indication: Acute migraines
- Date: August (estimated)
RedHill’s initial application filed in 2013 was met with a FDA complete response letter issued in Feb. 2014, citing concerns regarding third-party chemistry, manufacturing and control, and about the labeling and packaging. The company made a resubmission last November, and the resubmitted application was deemed complete.
The PDUFA date was set for the first half of 2018.
Allergan’s Uterine Fibroid Drug Awaits FDA Approval
- Company: Allergan plc AGN 0.53%
- Type of Application: NDA
- Candidate: Esmya
- Indication: Treating abnormal uterine bleeding in women with uterine fibroids
- Date: August (estimated)
The FDA extended the review period of Esmya, chemically ulipristal acetate, to August 2018 to provide time for a full review of the file.
Subscribe to:
Comments (Atom)