Bernstein analyst Vincent Chen started coverage on PTC Therapeutics (PTCT) with an Outperform rating and a $48 price target, given his positive view on the commercial potential of risdiplam in SMA. The analyst believes that there will still be a substantial market opportunity for non-gene therapy drugs after approval, and thinks risdiplam’s oral dosing will be a substantial advantage over Biogen (BIIB) Spinraza’s intrathecal administration. If risdiplam is safe and comparably efficacious to Spinraza, Chen forecasts potential for $2.5B in revenues by 2030.
Search This Blog
Thursday, April 11, 2019
Sandoz signs agreement with Shionogi for commercialization of Rizmoic
Sandoz, a Novartis division, announces that it has signed an agreement with Shionogi for commercialization of Rizmoic in the markets of Germany, the UK, and the Netherlands, plus right of first refusal for certain other European markets. Rizmoic, a once-daily 200-microgram oral tablet discovered and developed by Shionogi, is a medicine indicated to treat opioid induced constipation, or OIC, in adult patients previously treated with a laxative. Shionogi announced on February 22 that it had received EU marketing authorization for Rizmoic. Under the terms of the agreement, Sandoz will be responsible for commercializing Rizmoic in Germany, the UK, and the Netherlands and Shionogi will be responsible for its manufacturing and development. Further details of the deal are not being disclosed.
Guardant Health Liquid Biopsy Test to be Covered by EviCore
Guardant Health said Wednesday that its Guardant360 liquid biopsy test will be covered by health plans associated with technology assessment firm EviCore beginning this summer.
As a result, the test will have become a covered benefit for approximately 150 million people in total.
The new decision by EviCore will make the test covered for its health plan members as medically necessary to assist in selecting therapy for patients with advanced lung cancer, beginning July 1. Guardant said the new decision followed the University of Pennsylvania prospective study publication in JAMA Oncology, which evaluated the use of Guardant360 to guide the first-line treatment of patients with advanced lung cancer. Investigators in that study found that Guardant360 has sensitivity and detection rates comparable to tissue testing, and that patients responded to the treatment selected based on Guardant360 results.
Guardant said the EviCore decision — coupled with previous payor endorsements by Health Care Service Corporation, Independence Blue Cross, Highmark Blue Cross Blue Shield, Blue Cross Blue Shield of Tennessee, and Government Employees Health Association/Federal Employees Program — represents an addition of 38 million covered lives in 2019.
The company estimated that 115 million lives were covered by prior payor decisions for Guardant360.
According to Guardant, its test has already been ordered by more than 6,000 oncologists for more than 80,000 patients with advanced cancer since its introduction in 2014. The company did not break down how many of these orders have been for payor-covered lung cancer indications.
Genome-Scale CRISPR Screening Helps Identify New Cancer Drug Targets
A team led by researchers at the Wellcome Sanger Institute has used genome-scale CRISPR screening to create a new resource of cancer dependencies and develop a framework to prioritize existing cancer drug targets and suggest new ones.
As they reported today in Nature, the researchers performed CRISPR-Cas9 screens in more than 300 human cancer cell lines from 30 cancer types. They integrated cell fitness effects with genomic biomarkers and target tractability for drug development to systematically prioritize new targets in defined tissues and for specific genotypes. Using this method, they were able to verify the Werner syndrome ATP-dependent helicase (WRN) as a synthetic lethal target in tumors from multiple cancer types with microsatellite instability.
“The principles described in this study can inform the initial stages of drug development by contributing to a new, diverse, and more effective portfolio of cancer drug targets,” the authors wrote.
To comprehensively catalogue genes that are required for cancer cell fitness, the team began by performing 941 CRISPR fitness screens in 339 cancer cell lines, targeting 18,009 genes. After quality controls, the final analysis set included 324 cell lines from 30 different cancer types across 19 different tissues, ranging from common cancers — such as lung, colon and breast cancer — to cancers of unmet clinical need — such as lung and pancreatic cancer.
The researchers also identified a median of 1,459 fitness genes in each cell line. In total, 41 percent of all targeted genes had a fitness effect in one or more cell lines and 83 percent of these genes induced a dependency in less than 50 percent of the tested cell lines.
In order to identify core fitness genes, they then developed a statistical method called the adaptive daisy model, or ADaM. Genes that were defined as core fitness in at least 12 out of 13 cancer types were classified as pan-cancer core fitness genes. This analysis yielded a median of 866 cancer-type-specific and 553 pan-cancer core fitness genes.
Of the pan-cancer core fitness genes the researchers identified using ADaM, 399 were previously defined as essential genes. They also found that 125 of these genes are involved in essential cellular processes. The remaining 132 genes were newly identified and are also significantly enriched in cellular housekeeping genes and pathways.
The team noted that blood cancer cell lines had the most distinctive profile of core fitness genes. It further found that cancer-type-specific core fitness genes were generally highly expressed in matched healthy tissues, consistent with their predicted role in fundamental cellular processes. This suggested that they could show potential toxicity if used as targets.
“Overall, using a statistical approach, we refined and expanded our current knowledge of core fitness genes in humans and identified genes that have a high likelihood of toxicity, which thus represent less favorable therapeutic targets,” the authors wrote. “Furthermore, owing to the large scale of our dataset, we could now define context-specific fitness genes, many of which had a loss-of-fitness effect that was similar to or stronger than core fitness genes.”
In order to winnow the list of context-specific fitness genes down to a list of promising therapeutic targets, the researchers developed a computational framework to assign each gene a target priority score — excluding genes that were likely to be poor targets because of potential toxicity, core fitness genes, and genes that were not expressed or homozygously deleted. For each gene, 70 percent of the priority score was derived from CRISPR experimental evidence and averaged across dependent cell lines on the basis of the fitness effect size, the significance of fitness deficiency, target gene expression, target mutational status, and evidence for other fitness genes in the same pathway. The remaining 30 percent of the priority score was based on evidence of a genetic biomarker that was associated with a target dependency and the frequency at which the target was somatically altered in patients’ tumors.
“In total, we identified 628 unique priority targets, including 92 pan-cancer and 617 cancer-type-specific targets. The number of priority targets varied approximately threefold across cancer types with a median of 88 targets,” the authors wrote. “The majority of cancer-type priority targets (74 percent) were identified in only one or two cancer types, underscoring their context specificity. Most priority pan-cancer targets (88 percent) were also identified in the cancer-type-specific analyses.”
The team then split these targets into three groups according to their tractability, with group 1 comprised of targets of approved anticancer drugs or compounds in clinical or preclinical development, group 2 containing targets without drugs in clinical development but with evidence that supports target tractability, and group 3 including targets that had no support or a lack of information that could inform tractability.
“Priority targets in tractability group 1 were enriched in protein kinases, highlighting a major focus of drug development against this class of targets, compared to groups 2 and 3, which included a more functionally diverse set of targets,” the team noted. “Targets in group 2 are most likely to be novel and tractable through conventional modalities and, therefore, represent good candidates for drug development.”
Importantly, the researchers added, this target prioritization strategy confirmed the WRN helicase as a promising target in MSI cancers. WRN has diverse roles in DNA repair, replication, transcription, and telomere maintenance. The team’s findings showed that the helicase is necessary to sustain in vivo growth of colorectal cancer cells with MSI.
“New approaches are needed to effectively prioritize candidate therapeutic targets for cancer treatments,” the authors concluded. “Confirmatory studies are necessary to further evaluate the priority targets that we identified. Even a modest improvement in drug-development success rates, and an expanded repertoire of targets, through approaches such as ours could provide benefits to patients with cancer.”
Deadly ‘Super Fungus’ Fuels Surge in Chinese Drugmakers’ Shares
A deadly fungus that is spreading across the globe is sending Chinese pharma stocks surging.
Investors rushed to buy shares in biomedical companies in the mainland and Hong Kong after media reported that China has confirmed at least 18 cases of infection “in recent years” by the Candida auris fungus highlighted in an April 6 New York Times story for its drug resistance. Shandong Sinobioway Biomedicine Co. has rallied 27 percent in the past three days.
“Super fungus is a hot topic in China now. No medicine is able to cure the super fungus, but still that’s enough to be a trigger for some hot-money flow into the pharma sector,” Zhang Gang, Shanghai-based strategist at Central China Securities Co., said by phone.
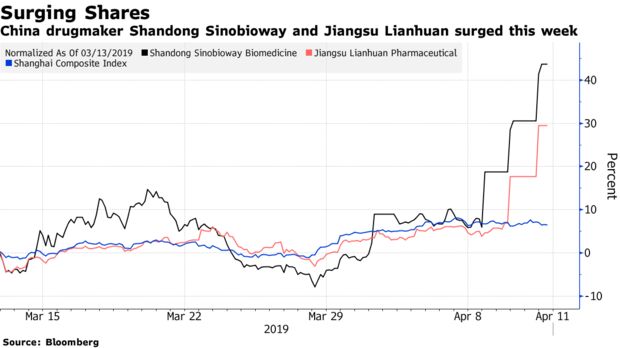
Other drugmakers that have risen by their 10 percent limits in the past few days include Jiangsu Lianhuan Pharmaceutical Co. and Jiangsu Sihuan Bioengineering Co. In Hong Kong, Shanghai Pharmaceuticals Holding Co. rose 2.8 percent to be among the best performers on the MSCI China Index. The benchmark Shanghai Composite Index closed down 1.6 percent.
Pharma stocks were one of the worst-performing sectors in China last year, hit by a government trial scheme that lowered prices of certain drugs significantly. Those shares have rallied after solid full-year earnings released in March, and the news of the fungus has provided a good catalyst for investors to add back their positions in the sector, Zhang said.
Angion Biomedica Nears End of Clinical Trials for Organ-Repairing Molecule
Repairing a damaged organ in order to allow an individual to have a healthier life, as well as a greater quality of life, could soon be a reality as New York-based Angion Biomedica Corp.moves toward completing clinical trials of its lead asset.
The company is developing ANG-3777, a small molecule harnessing the effects of Hepatocyte growth factor (HGF). HGF is an organ repair protein best known for providing the liver with a unique ability to regenerate itself. Angion research has noted that HGF also triggers extensive self-repair pathways within the kidneys, liver, heart, brain and lungs. Angion researchers discovered a small molecule that allows the body to drive a healing response.
Angion Chief Executive Officer Jay Venkatesan, who joined the company last year, said in a typical case when a patient has an injured organ, the body will attempt to repair itself. Angion’s lead candidate operates along the HGF pathway and “drives some self-repair along the natural pathways,” he told BioSpace in an exclusive interview. Currently, Venkatesan said there is no therapeutic on the market that delivers the same effect. Angion has begun its research with ANG-3777 in the kidney, and if it continues to demonstrate efficacy and safety in the late-stage trial, Venkatesan said the company will likely explore its uses in other organs.
“Right now it seems to be a ubiquitous path,” Venkatesan said. That means that the Angion candidate could drive a healing response in almost any organ in the body. “It’s interesting to see what the limits are.”
AGN-377 is in two studies, a Phase II and a Phase III. The late-stage study concerns patients who have undergone a kidney transplant. The trial will continue a Phase II study of kidney transplant patients who had low or no urine output post-transplant. In the Phase II trial, patients were provided three doses of ANG-3777 within three days of the transplant. Over an assessed period of 28 days, the patients demonstrated increased urine output, lowered serum creatinine and improved renal function. Additionally, the results showed that the dosing reduced the need for dialysis. The Phase II observations, which Venkatesan said was meant to be a proof-of-concept study, will be confirmed in the Phase III GIFT (Graft Improvement Following Transplantation) study. In the late-stage trial, patients will be provided three doses of ANG-3777 or placebo within 30 hours of transplant. The trial will include about 300 people. Data from this trial is expected in the first quarter of 2020.
The data was compelling enough for Vivek Ramaswamy’s Sinovant to license AGN-3777 for potential commercialization in the People’s Republic of China, Hong Kong, Macau and Taiwan. Sinovant will conduct clinical trials of the asset, which it dubbed BB3, in China.
Angion is also conducting a mid-stage trial in cardiac patients. Data from this trial is expected in the second quarter next year. Angion plans to initiate a heart attack study as well in order to improve measure of heart function following catheterization.
ANG-377 isn’t the only exciting asset in Angion’s pipeline. Last week the company secured a $4.76 million Department of Defense follow-on grant for ANG-3070, the company’s precision drug candidate for the treatment of fibrotic diseases. Specifically, ANG-3070 is in development for primary focal segmental glomerulosclerosis (FSGS), a form of chronic kidney disease.
The DoD grant supports a previous $2.2 million grant that was used to establish preclinical proof-of-concept for ANG-3070 as an anti-fibrotic agent with the potential to delay or halt the progression of FSGS. Angion partnered with Nephrotic Syndrome Study Network to advance the study of ANG-3070. The study data is expected to support the completion of an Investigational New Drug Application and launch human studies of ANG-3070 for FGS and other fibrotic conditions. Venkatesan anticipates the potential start of Phase I trials in the third quarter of this year and the beginning of efficacy studies in 2020.
As Angion pushes forward with the development of its candidates, the company is also pushing forward in growth. Venkatesan said the company will likely double in headcount by the end of the year.
“I’ve been happy with the progress we’re making with 3777 and pushing 3070 into the clinic. Once that gets into the clinic, there’s a lot of places we can go with that,” Venkatesan said.
Gilead Presents New NASH Data at the International Liver Congress
– New Proof-of-Concept Data for Combination of Investigational FXR Agonist Cilofexor and ACC Inhibitor Firsocostat in NASH —
Gilead Sciences, Inc. (Nasdaq: GILD) today announced new data from the company’s clinical research program in nonalcoholic steatohepatitis (NASH) being presented at The International Liver Congress™ 2019 in Vienna. The data support Gilead’s efforts to develop combination therapies to target different aspects of NASH, evaluate the utility of noninvasive tests for the identification of patients living with the disease and advance overall understanding of the complexities and burden of NASH.
“NASH is a complex disease with multiple biological pathways that influence its progression. Combination therapeutic approaches which target these pathways, are likely to be needed to effectively treat patients living with NASH, particularly those with advanced fibrosis who have the greatest unmet need,” said John McHutchison, AO, MD, Chief Scientific Officer, Head of Research and Development, Gilead Sciences. “We are encouraged by the results of our proof-of-concept study being presented this week and look forward to sharing further combination data from the Phase 2 ATLAS trial later this year.”
Combination Therapy Treatment for NASH
A proof-of-concept study demonstrated that the combination of the investigational, selective, non-steroidal farnesoid X receptor (FXR) agonist cilofexor (GS-9674) and the acetyl-CoA carboxylase (ACC) inhibitor firsocostat (GS-0976) resulted in improvements in hepatic steatosis, liver stiffness, liver biochemistry and serum fibrosis markers. In the study, 20 patients with NASH received cilofexor 30 mg and firsocostat 20 mg orally once daily for 12 weeks. A significant decline of at least 30 percent in hepatic fat measured by magnetic resonance imaging-proton density fat fraction (MRI-PDFF) from baseline to 12 weeks was observed in 74 percent of patients. Improvements in liver biochemistry tests including serum ALT (median relative reduction, -37%; p<0.001) and GGT (-32%; p<0.001), along with markers of reduced bile acid synthesis, were observed at 12 weeks. Treatment was well tolerated and pruritus was not reported in any patients. Asymptomatic, Grade 3 hypertriglyceridemia was observed in two patients. No patients withdrew from the study due to adverse events.
Subscribe to:
Posts (Atom)