Atopic dermatitis is an inflammatory disease of the skin in which the normal barrier function of the skin is disrupted, so that immune function is impaired and susceptibility to infection with
common bacteria like Staphylococcus aureus is increased.
This invasion by pathogenic microbes in turn leads to a disturbance in the numbers and relative proportions of various microbial species that live as commensal organisms on the skin, causing cutaneous dysbiosis.
Why is eczema treatment so urgent?
Atopic dermatitis is a common skin disorder, affecting about one in five children in developed nations. Moreover, it can profoundly disturb the individual’s quality of life and healthcare expenditures. Furthermore, atopic dermatitis is associated with
development of asthma,
allergic rhinitis, and
food allergies. Children will often not cooperate with the multiple applications and oral medications that make up its treatment at present, and either way, their quality of life is decreased. More effective biologics are now available but the cost is still steep, putting it out of reach in many places.
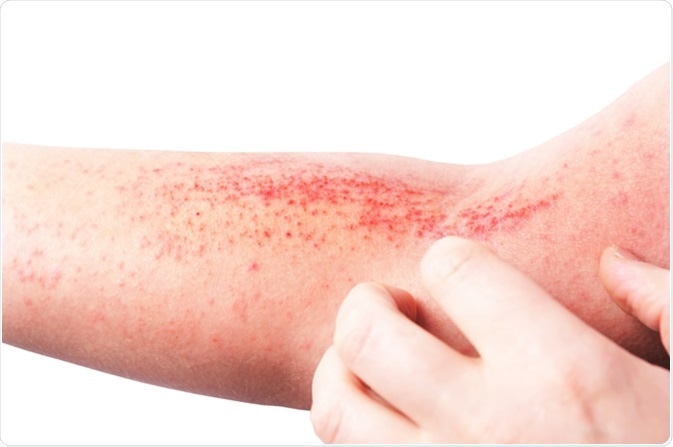
Atopic dermatitis (AD), also known as atopic eczema. Image Credit: LIAL / Shutterstock
Its incidence is shooting up in more developed nations, but most affected people will experience a marked reduction in symptoms around the time of puberty.
The chronic risks do not stop here, however, for the long duration during which the skin fails to provide a proper barrier to the invasion of foreign microbes and the removal of moisture takes its toll in the subsequent development of a hypersensitive skin which reacts to many other antigens.
How does the skin microbiota affect eczema?
Theoretically, topical and preventive oral antibiotics against S. aureus should be effective in treating atopic dermatitis. However, many studies have shown the reverse, so antimicrobial treatment are not part of standard treatment. It may be that there are more microbes to consider than just S. aureus (a gram-positive bacterium) in the pathogenesis of this disorder. Gram-negative bacteria on the skin are lower in skin areas most commonly affected by eczema compared to healthy persons.
Earlier studies have shown how important the skin microbiome is in eczema. Mapping the locations of skin Gram-negative bacteria revealed a stunning overlap with the sites where eczema typically breaks out – the antecubital fossa (front of the elbow) and the back of the knees, for instance. This suggests that when the wrong strains of certain bacteria replace the beneficial ones, atopic dermatitis may result. This led to scientists trying to correct the skin microbiome to treat eczema.
Spraying skin with bacteria successfully treats eczema
A recent experimental study used a Gram-negative commensal bacterium, Roseomonas mucosa, taken from the skin of healthy volunteers as well as from people with the condition. This microbe was studied on cell cultures and in mice.
Interestingly, in cell cultures R. mucosa killed S. aureus, while in mice it enhanced the skin’s barrier function, keeping it hydrated and preventing the entry of pathogens. Moreover, it also improved atopic dermatitis rashes in mice.
In a small human trial, the treatment improved the immune status of skin cells. However, the R. mucosa cultured from the skin of people with atopic dermatitis had a negative or no impact on the rashes.
During the first part of the trial, designed to look at the effectiveness and safety of the therapy, a mix of three live R. mucosa strains collected from healthy volunteers, dispersed in 10% -15% sucrose solution (sugar water), was sprayed on two skin sites, one in the front of the elbow and the other site chosen by the patient) by the adult subjects in preset increasing doses, from 103 to 105 colony-forming units (CFU) at each treatment site, twice a week for 6 weeks.
In the second part of the trial, five children were enrolled and treated with the same dose regimen for 12 weeks. From weeks 13-16, the treatment was escalated to once every alternate day.
Important findings
The findings of this trial, early though it is, were remarkable in some ways. For one, the administration of R. mucosa in mice was not associated with any toxicity, and there were no reported problems or adverse effects in humans either. The investigators found that both patient symptoms like itching and objective signs of the disease were significantly reduced. Steroid applications were decreased.
Secondly, the present trial achieved a treatment response beyond the minimum required effect level that is statistically significant for treatment effect, that is, >50% improvement in the SCORAD. This is in comparison to the historical placebo effect of 5% to 30%.
In children treated with topical probiotics, this level of improvement was seen in almost 80% of patients; 85% of treated adults also improved.
The overall number of patients who showed clinical improvement (68%) was greater than the 27% who would normally be expected to improve in accordance with the natural history of the disease.
All responders showed continued benefit even after the washout phase, with some manifesting additional improvement.
Strain-specific R. mucosal metabolites can promote dysbiosis in atopic dermatitis by decreased production of phospholipids which damage the epithelial barrier, compounds like histidinol, and monomethylgluterate, which irritate the epithelium, and reduce skin immunity. Thus providing topical “good” strains of R. mucosa could be of benefit to humans with atopic dermatitis in all these aspects.
The growth of beneficial R. mucosa is also affected by skin exposure to compounds like parabens which are commonly used as preservatives in soaps and skin products, but not that of S. aureus or R. mucosa from diseased skin. On the other hand, dilute bleach selectively inhibited S. aureus and R. mucosa from diseased individuals, but not from healthy volunteers.
Which patients are less likely to respond?
Responders were less likely than non-responders to have a family history of adult atopic dermatitis or at least three generations affected by atopic dermatitis.
Conclusion
This small preliminary study suggests several implications.
Different strains of R. mucosa produce different effects on the skin in patients with atopic dermatitis. When taken from normal volunteers with healthy skin, these organisms may produce clinically significant benefit in terms of improvement of skin rashes and itching, which is not seen when the organisms is isolated from the unaffected patches of skin in individuals with eczema.
The topical application of the bacteria may help restore the epithelial barrier function, correct the imbalance between innate and adaptive immunity, and restrict the growth of S. aureus leading to a lower population of this organism.
Genetic factors may also affect the response to treatment using R. mucosa.
Certain skin products may worsen atopic dermatitis by affecting the colonization of beneficial R. mucosa strains but not of pathogenic strains of S. aureus.
The next step will be to conduct a placebo-controlled trial for patients with atopic dermatitis to confirm or disprove these findings.
