Still, it shows that even in the earliest stages of the business closures that have since spread across the country, the cuts were most heavily felt in industries such as hotels, restaurants and education as the travel industry shut down, bars and eateries closed their doors, and day care centers shuttered, all in the aim of limiting the spread of the disease.
And, perhaps ironically in the middle of a health crisis, the health care sector was among the most afflicted as providers of nearly any service apart from acute care for sufferers of COVID-19, the lung ailment caused by the novel coronavirus, suspended operations and stopped seeing patients.
The following charts offer a picture of how March’s job losses – certain to be revised higher and followed by even larger cuts in April – played out across various industries and demographic groups.
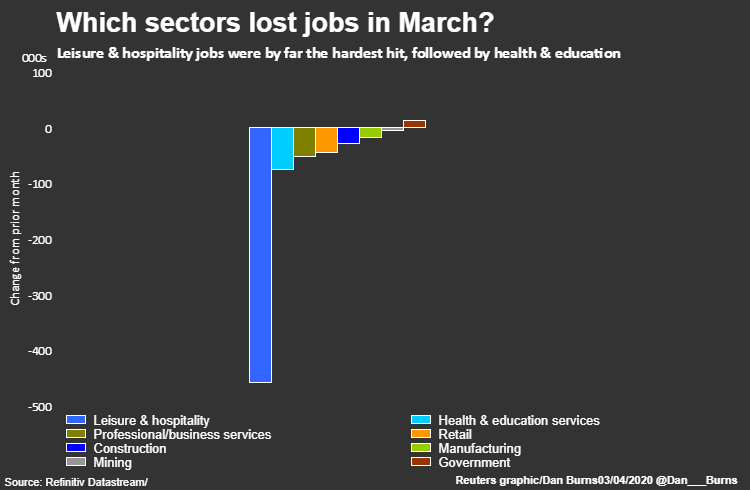
The leisure and hospitality sector shed 459,000 jobs – 65% of all the positions lost in March. The loss, the largest monthly decline in the sector ever, effectively wiped out two years of employment gains in the industry.
The largest share of that came at restaurants and bars, which slashed 417,000 jobs.
Around 76,000 health and education jobs were eliminated led by 29,000 cuts at dentists and physicians offices and another 19,000 at day care centers.
The federal government sector stood out as a rare example of net job gains last month, thanks to the addition of 17,000 temporary workers for the 2020 census.

The unemployment rate shot up to 4.4% from a half-century low of 3.5%, the largest one-month increase in the jobless rate since 1975.
By race or ethnicity, the largest increases were seen among Asians and Latinos, with increases of 1.6 percentage points each, nearly twice the overall increase of 0.9 percentage point. Both whites and African Americans saw their rates rise at the same pace as the national rate, although the unemployment rate now for blacks – at 6.7% – is 65% higher than for whites at 4%.
The youngest workers were also the most likely to lose work in the early stages of the shutdown.
The unemployment rate for teenagers rose by 3.3 percentage points to 14.3% and for those between 20 and 24 years old by 2.3 points – the most since 1953 – to 8.7%.
By contrast, unemployment for those in the 25-to-34-year-old age bracket rose by just 0.4 percentage point to 4.1%. The jobless rate for workers aged 45 to 54 rose 0.7 percentage point to 3.2%, the lowest rate for any age group.

Workers with lower levels of education also found themselves thrown out of work at a higher rate in March.
The rate for workers without a high school diploma jumped by 1.1 percentage points to 6.8%, the highest in nearly three years.
For people with a college degree, meanwhile, the jobless rate rose by 0.6 percentage point to 2.5%. Still, it was the largest monthly increase in the rate for that demographic since the Labor Department began tracking it in the early 1990s.
The overall rate for both sexes over the age of 20 now stands at 4%.
https://www.reuters.com/article/us-health-coronavirus-usa-jobs/how-the-coronavirus-job-cuts-played-out-by-sector-and-demographics-idUSKBN21M0EL
