As the push for some to head back to the office continues, despite being the midst of the ongoing pandemic, a focus has shifted to what kinds of technologies are going to be used in order to make sure that public areas, like office environments and airports, are virus-free.
Aside from the usual hand sanitizers and face masks,
FT published a report this week highlighting some of the other technologies that businesses are choosing to employ in order to surfaces and spaces clean.
Shaun Fitzgerald, visiting professor at the University of Cambridge, said: “Pandemics like this can provide fertile ground for creative minds to think about how to do things differently.”
One option that’s being looked at is self-cleaning surfaces. While the virus can stay alive for up to 72 hours on plastics and steel, silver and copper have a track record of killing viruses and bacteria within four hours. Felicity de Cogan, research fellow at the University of Birmingham and founder of NitroPep, said that timeframe needs to get down to “seconds to minutes” and it needs to be “built into the material”.
Her company is working on developing layers of materials with spike particles on them that puncture and kill viruses within minutes. Her company’s antimicrobial agents can be added to already existing desks, walls and other surfaces and rupture “anything with a membrane”.
“It doesn’t require a change in behaviour, it just sits there and kills whatever lands on it,” de Cogan said. In a pilot run of the technology for a Royal Navy ship, it killed more than 95% of bacteria like E. Coli and MRSA. de Cogan aims to implement it in public transport and for self-cleaning equipment, if it proven to be able to kill coronavirus.
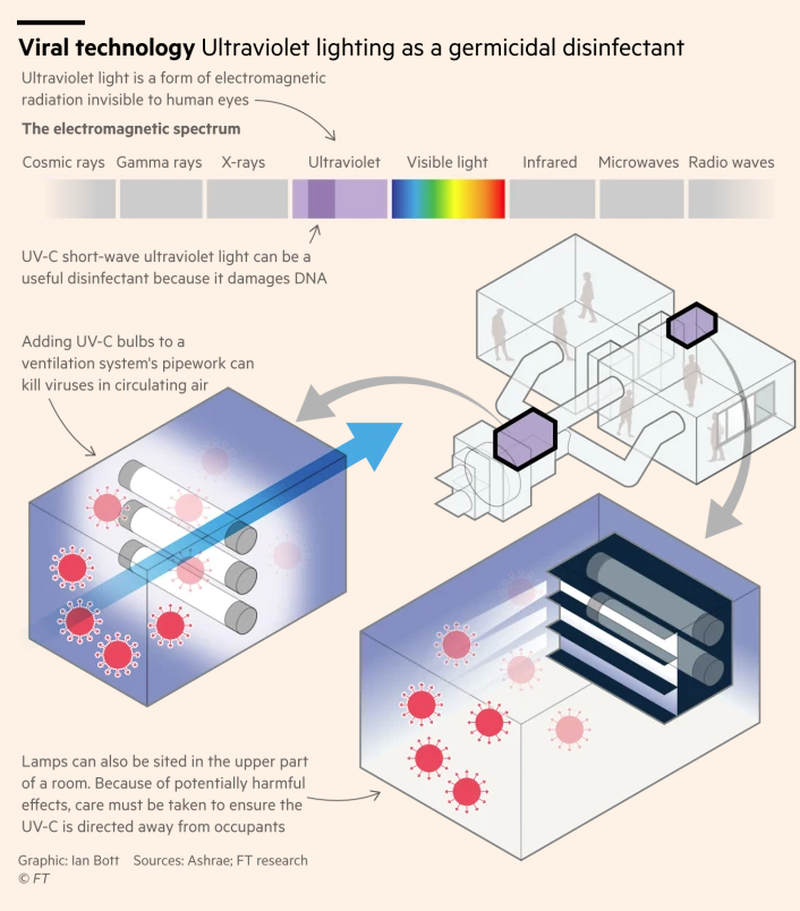
Another option is UV irradiation. The idea of “germicidal ultraviolet” has been known for years but is now getting another look. UV beams are used to kill micro-organisms by targeting the RNA in viruses and DNA in bacteria and fungi. UV lamps have been found in the past to be effective in stopping drug-resistant tuberculosis in large rooms.
The technology is good for large rooms and crowded and poorly ventilated environments. Covid has accelerated demand for UV charging robots that emit UV light that leaves bacteria and viruses too damaged to function. They robots cost about 60,000 Euro each and can be found in places like hospitals and hotels.
A third technology being used is environmental monitors that “check the pulse” of a building that already exists to assess things like CO2 levels. In Switzerland, researchers are trying to develop sensors that detect the virus itself. The Swiss Federal Institute of Technology (ETH Zurich) and Swiss Federal Laboratories for Materials Science and Technology (Empa) have together developed a sensor inside of a chamber that emits light if it finds the viruses RNA. Real-world testing is set to start soon.
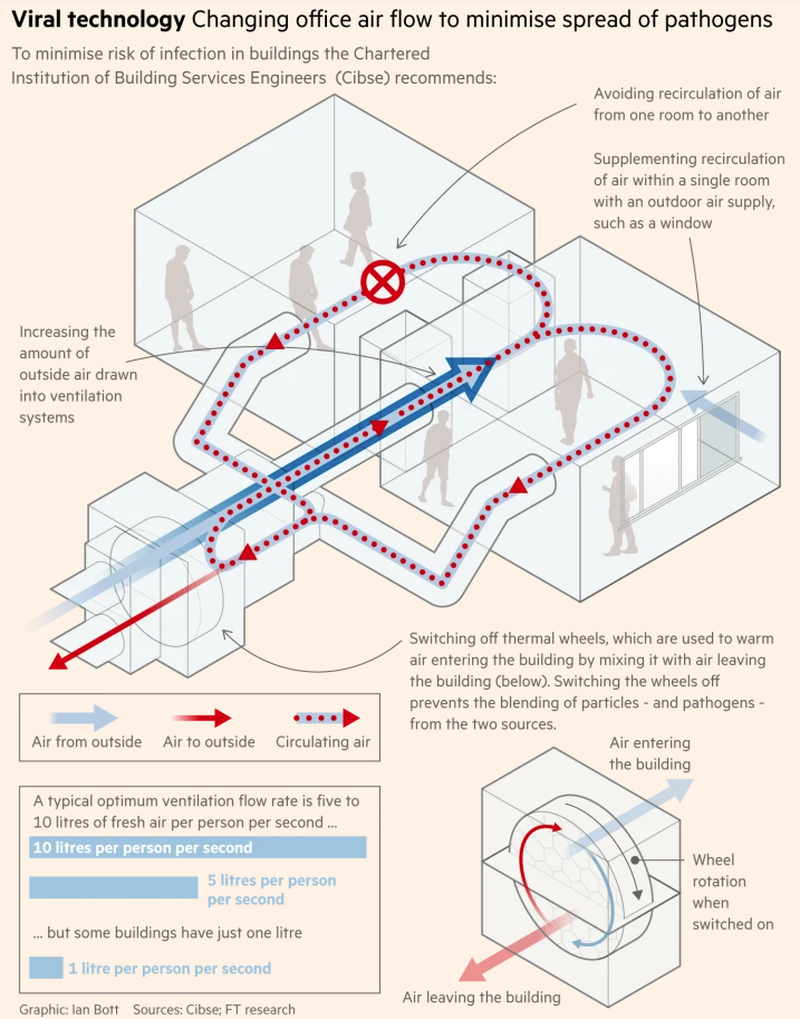
Finally, ventilation is in focus. HVAC systems play a huge role in the accumulation of aerosol droplets and improving the amount of fresh air a person gets per second can help slow the viruses movement. This can be especially true in confined spaces like elevators and airplanes.
Gardner Allen, assistant professor at the Harvard TH Chan School of Public Health said: “You always want the air to move from clean to dirty and then out. In the bathroom, you want it to move from indoor, to bathroom and then out through the exhaust.”
One thing is for certain: the world is changing and evolving to try and deal with the pandemic threat in ways that 6 months ago, we would have never thought possible. Surely this will lead to a whole host of new Silicon Valley startups that will focus not only on combating the virus, but also likely on burning cash at ungodly WeWork-style levels.
Keep your eyes out for those “exciting” IPO opportunities to probably start hitting the market around late 2021.