Update 10:55am: Here is another reason to be optimistic - not suicidal - by the news that the Omicron variant is spreading. As we, Ackman and Kolanovic, all said, the Omicron variant could be the best thing that happened to the pandemic as it is much more "mild" than Delta, and because it is much more transmissible could soon become the dominant version.
As the Minnesota Dept of Health said in its press release on the second identified US covid case, the "Infected Minnesota resident recently returned from domestic travel; variant was found through MDH variant surveillance program, which is one of the strongest surveillance programs in the nation"
And the punchline:
"The person with the Omicron variant is an adult male, is a resident of Hennepin County, and had been vaccinated. The person developed mild symptoms on Nov. 22 and sought COVID-19 testing on Nov. 24. The person’s symptoms have resolved."
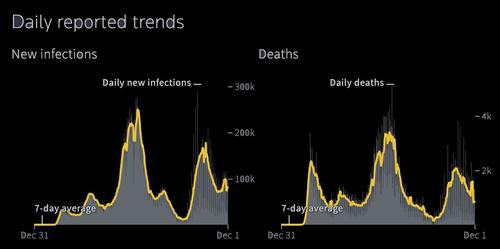
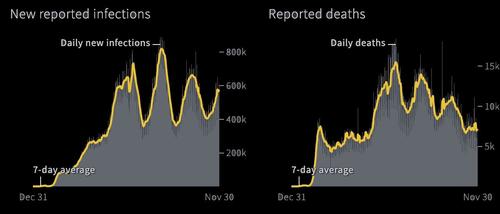
In other words, just as we noted, Omicron continues to manifest itself in an extremely mild fashion, and its symptoms go away in days (it is unclear how it progresses in unvaccinated people so far).
We also know what happens next: according to the press release...
The person spoke with MDH case investigators and reported traveling to New York City and attended the Anime NYC 2021 convention at the Javits Center from Nov. 19-21. The person was advised to isolate from others. Minnesota epidemiologists will continue to investigate in collaboration with New York City and the U.S. Centers for Disease Control and Prevention.
Said otherwise, expect a cluster of Omicron cases in NYC in the next 24-48 hours. And more importantly, with physicians hoping to get an accurate picture of the symptoms of the new strain, we should have a clear picture of just how benign Omicron is within the next 72 or so hours.
* * *
Earlier
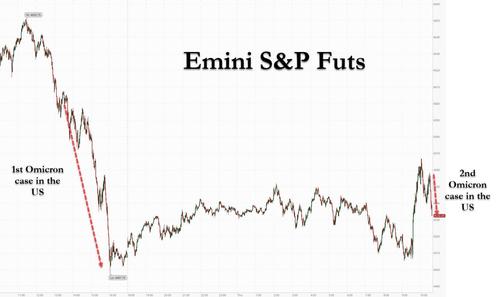
The first time that algos dumped everything on news that an Omicron case had been identified in the US in San Francisco, that was at least somewhat understandable even if virtually everyone knew it was just a matter of time before the "extremely transmissible" Omicron made its way to the US. However, when moments ago Reuters reported that a second Omicron cade had been identified in Minnesota...
- OMICRON COVID-19 VARIANT CASE IDENTIFIED IN MINNESOTA -STATE HEALTH DEPARTMENT
... for algos to pull off the same exact stunt was borderline stupid - after all, every incremental omicron case brings us closer to trillions in stimmies which as everyone by now knows send stocks soaring (inflation will be ignored by everyone if stonks are surging faster than prices are rising). And yet, the dump we just observed is precisely the same that took place yesterday, prompting some - such as us - to ask just what 13-year-olds are programming these algos, that trade the market in the dumbest possible way.
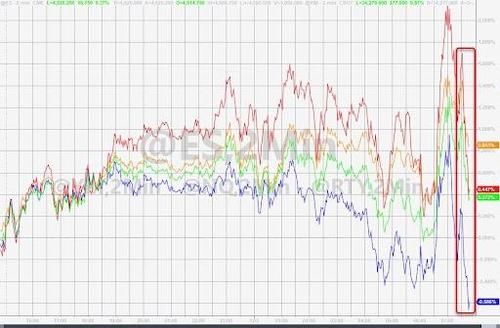
It wasn't just stocks: everything tumbled - yields, cryptos, you name it...
So for all those confused what they should be doing here, let us repeat, what we have been saying and what Rabobank said just moments ago - Omicron "will of course be used as an argument for more stimulus ahead." Those selling stocks on this news clearly have not been paying any attention to what happened in the past year.