Zhongzhi Enterprise Group Co., also called "China's Blackstone," which once oversaw more than 1 trillion yuan ($140 billion) before the property crisis, filed an application for bankruptcy in Beijing's First Intermediate People's Court, according to Bloomberg.

Zhongzhi wrote in a filing that it "obviously" could not repay its debts. A letter to shareholders in November explained its debts total 420 billion yuan to 460 billion yuan ($64.4 billion), compared with assets of 200 billion yuan.
On the Chinese social media platform WeChat, the Beijing court published a statement saying that Zhongzhi's "assets are insufficient to pay off all debts, and it clearly lacks the ability to repay in full."
Zhongzhi was once the most prominent firm in the country's $3 trillion trust industry, which pools savings from wealthy households and corporate clients to make loans and invest in real estate, stocks, bonds, and commodities. The bank first came on our radar last August when it skipped payments on several investment products, sparking rare protests outside its buildings by angry investors.
In November, Chinese authorities opened a criminal investigation into the firm's management business after revealing a $36.4 billion hole in its balance sheet.
"The persistent decline in the real estate market, coupled with stringent policies and increased financial anti-corruption measures, has hindered timely asset collection," said Zhao Jian, head of the Atlantis Financial Research Institute in Beijing.
Jian warned: "Redeeming these assets has become exceedingly challenging."
According to Bloomberg, the trust sector's exposure to real estate is about 2.2 trillion yuan, or 10% of total assets as of the end of 2022.
"The big danger is that a negative feedback loop kicks in, with property stress causing strains in the financial system, undermining credit expansion and depressing growth, which, in turn, exacerbates the slump in the property sector," Bloomberg Economics wrote in a recent note.
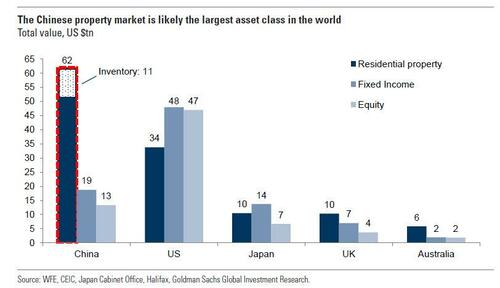
The latest developments at Zhongzhi stoke worries about contagion amid China's deflationary pressures and, of course, a struggling property market, which, as a reminder, is the largest asset class on earth...
It's an age-old dilemma for the central bank, faced with a weakening economy and an imploding property market. In November, the People's Bank of China planned to unleash a trillion yuan in low-cost funding to shore up property markets, hoping to stabilize the downward spiral.
https://www.zerohedge.com/markets/chinas-shadow-banking-giant-file-bankruptcy