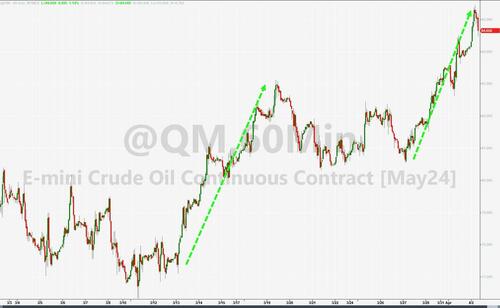
As discussed in our morning wrap, US equity futures are dipping lower as bond yields in the US continue to move higher as crude continues to surge and is up another 2% on growing fears of middle-eastern escalation after a senior Iranian commander was killed by an Israeli airstrike in Syria yesterday, with Iran immediately vowing revenge, and as Ukraine once again struck oil infrastructure targets deep inside Russia, overnight hitting Russia’s 3rd largest refinery, ~800 miles from the front lines.
As OilPrice details, Ukrainian drones hit the primary refining unit of Russia’s third-largest refinery southeast of Moscow more than 800 miles from the front line, Reuters reported on Tuesday. Ukraine keeps striking Russian oil assets despite the Biden admin's unequivocal demands for a hard stop, suggesting that diplomatic fallout is now imminent.
The Taneco refinery of Russian company Tatneft in Tatarstan, an industrialized region southeast of Moscow, was attacked by Ukrainian drones in the latest such attack from Ukraine on Russian refining infrastructure.
The refinery has a capacity to process 340,000 barrels per day (bpd) of crude. Its primary refining unit, with a capacity to process about 155,000 bpd, was hit in Tuesday’s attack, according to pictures seen by Reuters. The unit caught fire, which was swiftly extinguished, Russian media report.
They also quote Ramil Mullin, the mayor of the city of Nizhnekamsk, where the refinery is located, as saying that there have been no injured people in the attack.
“There are no injuries or serious damage,” Mullin wrote on Telegram. “The technological process of the enterprise has not been disrupted,” the mayor added.
A source with the Ukrainian intelligence in Kyiv told Reuters that Ukraine hit a major Russian oil facility in Tatarstan to reduce Russian oil revenues.
Ukraine has stepped up attacks on oil refineries in Russia in recent weeks, which have reduced Russian refining capacity, and which, reportedly, have the White House concerned about rising international prices.
The United States has repeatedly urged Ukraine to halt its drone attacks on Russian oil refineries due to Washington’s assessment that the strikes could lead to Russian retaliation and push up global oil prices, the Financial Times reported last month, citing sources familiar with the exchange.
According to Reuters estimates, the amount of Russian oil refining capacity that has been taken offline due to Ukrainian drone strikes is 14% of Russia’s total refining capacity.
Calculations show that 900,000 bpd of refining capacity have been taken offline by drone strikes, Reuters reported last week.
https://www.zerohedge.com/markets/ukrainian-drones-hit-russias-third-largest-oil-refinery