For years people have suspected that their smartphones, laptops, and home assistants were secretly eavesdropping on their private conversations. Now, a leaked pitch deck seems to confirm that our devices are, in fact, listening—and the implications are far-reaching.

A leaked pitch deck presentation from Cox Media Group (CMG) - major player in the digital advertising space, appears to detail how its "Active-Listening" software uses artificial intelligence to capture and analyze "real-time intent data" from users' conversations. The presentation, obtained by 404 Media, lays out how CMG's software could listen to everything spoken near a microphone-equipped device—whether it's a phone, laptop, or home assistant like Amazon's Alexa, the Daily Mail reports.
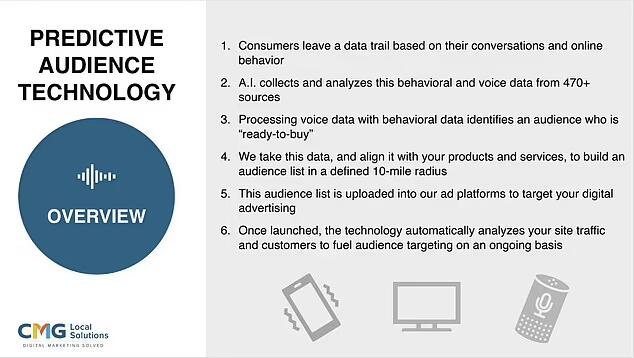
Active-Listening: A Six-Step Process to Harvest Voice Data
The pitch deck outlines a six-step process for CMG's Active-Listening software, which turns seemingly benign conversations into targeted advertising gold. The first slide describes how the software listens to your conversations and extracts real-time intent data, which advertisers can then pair with behavioral data to target "in-market consumers." In simple terms, it means that if you mention a product or service in a conversation, advertisers can target you with ads for that very item.
For instance, if you're talking about Toyota cars with a friend, you might soon find yourself inundated with ads for Toyota’s latest models. This alleged use of private conversations for ad targeting represents a significant breach of what most people consider to be their personal privacy.
The slideshow goes further, showcasing tech giants like Facebook, Google, and Amazon as clients of CMG, suggesting they may use this controversial technology. These companies have long denied listening to user conversations through device microphones, but this leaked document raises new questions.
Tech Giants in Hot Water: Denials, Uncertainties, and Potential Backpedaling
Following the leak, Google swiftly removed Cox Media Group from its "Partners Program" website, a move that suggests a distancing from the controversial practice. Meta, the parent company of Facebook, responded by stating it is reviewing CMG for any potential violations of its terms of service. Meanwhile, Amazon declared that its advertising division "has never worked with CMG on this program and has no plans to do so." However, their spokesperson left the door slightly ajar, stating that if a marketing partner violates their rules, action will be taken.
The mixed responses leave the status of these relationships somewhat murky. Are these denials simply a strategy to buy time while the companies reassess their partnerships with CMG, or are they genuinely unaware of these practices?
Is Active Listening Legal?
Although the notion of listening to conversations may seem like an egregious violation of privacy, CMG claims it is entirely legal. In a now-deleted blog post from November 2023, CMG wrote, "We know what you're thinking. Is this even legal? The short answer is: yes. It is legal for phones and devices to listen to you."
The legality, CMG argues, comes from the lengthy and often-overlooked "terms of use" agreements that consumers must accept when downloading new apps or updates. Buried in the fine print of these agreements, the software's Active Listening feature is sometimes included, making the practice legally permissible—if ethically questionable.
This might explain how CMG operates in states like California, where wiretapping laws typically prohibit recording someone without their knowledge. If users unknowingly consented to these practices when they accepted an app's terms of service, then companies like CMG can claim they are within their rights.
A Long-Running Speculation, Now Potentially Validated
For years, users have speculated that their phones or tablets were eavesdropping on their private conversations, only to serve up eerily targeted ads shortly after. Tech companies like Facebook, Google, and Amazon have consistently denied these claims. Meta’s online privacy center even states, "We understand that sometimes ads can be so specific, it seems like we must be listening to your conversations through your microphone, but we're not."
However, the leaked pitch deck paints a different picture. It appears to confirm what millions have long suspected: tech giants and their marketing partners could be cashing in on what users say in the privacy of their homes.
New Revelations Spark Further Concerns
This revelation follows a series of similar findings that have ignited debates over privacy in the digital age. Just a day after the CMG leak, 404 Media exposed another AI marketing company, MindSift, which boasted on a podcast about using smart device speakers to target ads.
As more details emerge, the extent of privacy violations continues to grow. The question now is, what steps will regulators, companies, and consumers take in response to these revelations? Will there be a move toward more stringent privacy protections, or will these practices become more ingrained in the digital advertising landscape?