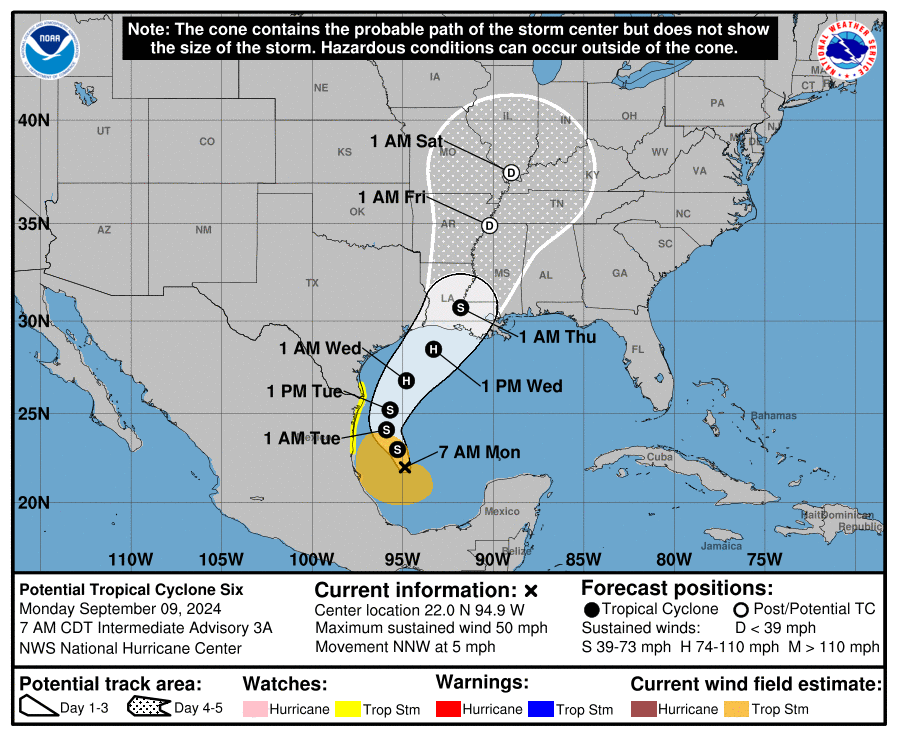
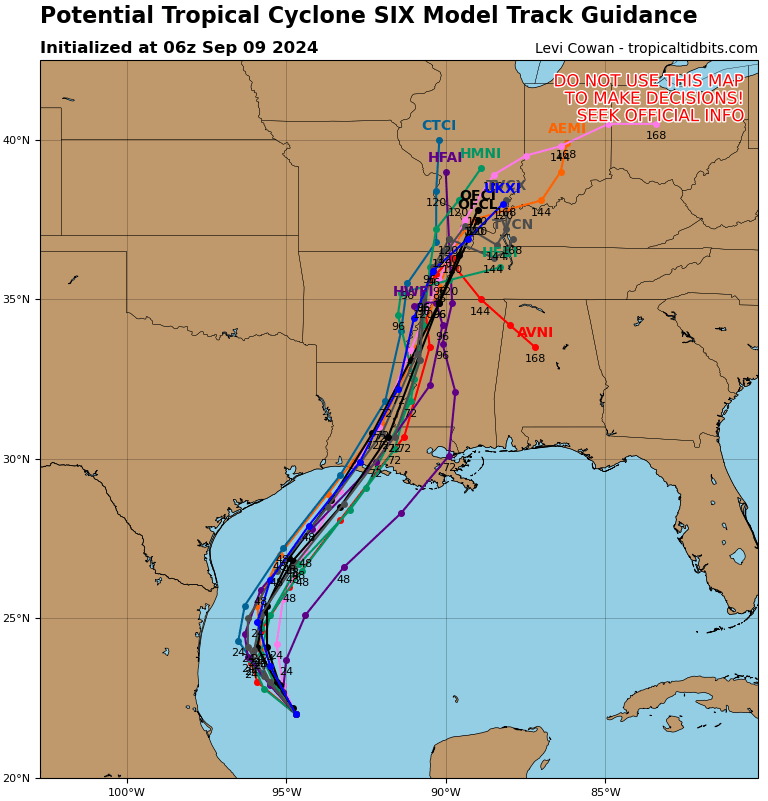
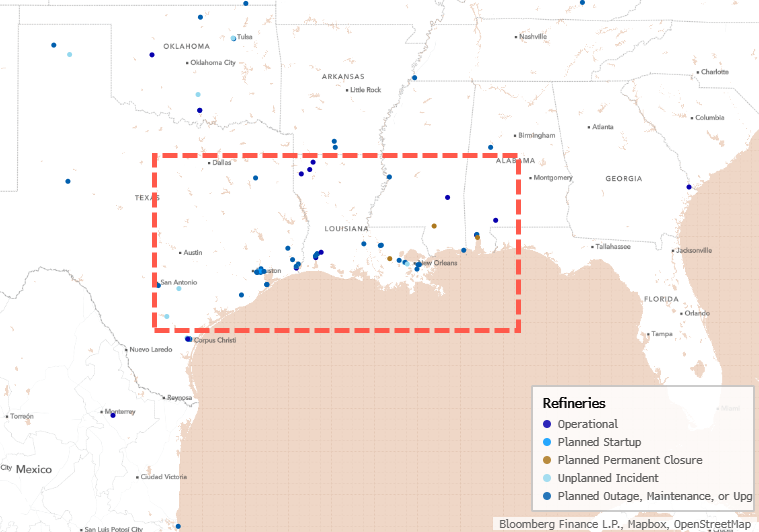
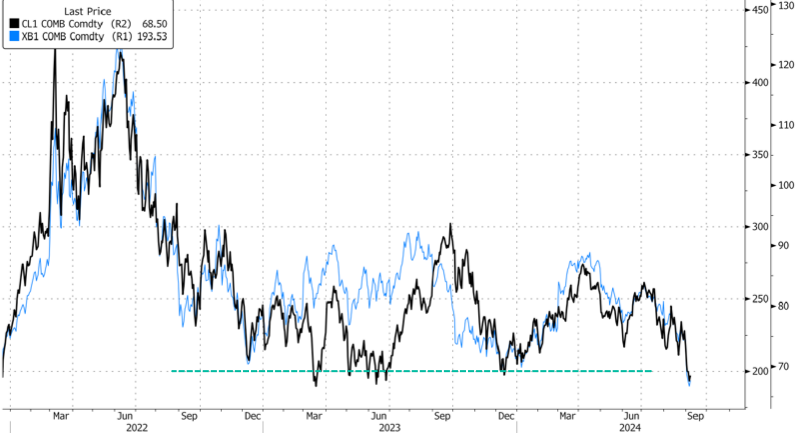
Over the past two years, Eisai and Biogen’s Leqembi and Eli Lilly’s Kisunla, both anti-amyloid antibodies, made history as the first real options to slow cognitive decline associated with Alzheimer’s disease. For years, amyloid plaques and tau tangles have been a primary target of Alzheimer’s disease research and drug development, but while affecting these proteins may yield some benefit, the illness continues to progress. Today, multiple therapeutics are in Phase III trials with other targets, suggesting that within the next few years it may become possible to treat Alzheimer’s via multiple pathways.
There are currently 32 Alzheimer’s therapeutics in 48 Phase III trials, according to a recent paper by neuroscientist Jeffrey Cummings, a professor at the University of Nevada. Six of those target amyloid-β and one targets tau, but the others aim to affect neuroprotection growth factors, neurotransmitters, neurogenesis, inflammation and proteinopathies. Of those, 21 are meant to be disease-modifying.
“Alzheimer’s disease is quite complex in its biology and diagnosis,” Rebecca Edelmayer, vice president of scientific engagement for the Alzheimer’s Association, told BioSpace. “We’re moving to a point where we have better understanding of the disease and what contributes to its symptomology, so those biological underpinnings become targets.”
These approaches won’t necessarily replace Leqembi and Kisunla, but they could increase therapeutic options to better manage the disease.
The First Anti-Amyloid Antibodies for Alzheimer’s
In January 2023, the FDA approved Leqembi as the second disease-modifying treatment for Alzheimer’s disease. The third, Kisunla, followed in July 2024. (The first drug, Eisai and Biogen’s Aduhelm, was discontinued earlier this year.)
While Leqembi and Kisunla are a positive step forward, experts suggest there is room for improvement. As measured by the Clinical Dementia Rating Sum of Boxes (CDR-SB), Leqembi reduced clinical decline by 27%, whereas Kisunla reduced clinical decline by 29%.
In addition to limited efficacy, the drugs both have notable side effects, specifically, the risk of amyloid-related imaging abnormalities (ARIA), the increase in lateral ventricular volume and the simultaneous loss of whole brain volume. A finding of ARIA on imaging tests is indicative of a brain bleed or swelling.
When physician online community Sermo questioned 268 neurologists, it found a diversity of opinion on how to best determine clinical benefit. Forty percent of those surveyed have treated Alzheimer’s patients with Leqembi or Kisunla.
“There’s debate about whether the two already approved therapeutics are that compelling, so the bar is low,” Andrew Tsai, senior vice president of equity research for the biopharma analyst Jefferies, told BioSpace.
A major difference between the two is that Leqembi is infused intravenously twice monthly, while Kisunla is infused only once per month. But any infusion is a pain point for many patients, not to mention a cost to the healthcare system, so easier administration is the goal for several next- gen Alzheimer’s therapeutics. Lilly’s drug is also designed to stop treatment once plaque clearance is achieved.
Phase III Alzheimer’s Candidates
Athira’s Fosgonimeton
Athira Pharma’s fosgonimeton is a small-molecule therapeutic that may be injected daily at home. The company recently reported data from a Phase II/III trial of the drug, which enhances signaling by neurotrophic hepatocyte growth factor (HGF).
The trial, which included 315 patients with mild-to-moderate Alzheimer’s disease, failed to meet its primary and key secondary endpoints regarding improvements in cognition and function at the 26-week point. However, pre-specified subgroups experienced rapid disease progression, while both cognition and function either stabilized or improved. Biomarkers also showed improvement, according to the company.
In preclinical models of Alzheimer’s disease, fosgonimeton has “been shown to reduce inflammation, improve mitochondrial and synaptic function and reduce amyloid toxicity and p-tau aggregation,” Chief Medical Officer Javier San Martin told BioSpace in an email.
“[While] Leqembi and Kisunla are monoclonal antibodies targeting removal of amyloid plaque from the brain with the expectation that by removing amyloid the neurons will be preserved . . . fosgonimeton focuses on improving neuronal health regardless whether the insult is amyloid, tau or other misfolded proteins,” he said.
Anavex’s ANAVEX 2-73
In New York, Anavex Life Sciences Corp. is targeting neuronal homeostasis in an ongoing Phase IIb/III study with blarcamesine, an oral, once-daily therapeutic that works “through autophagy through SIGMAR1 activation.”
“Blarcamesine’s ability to reduce pathological brain volume loss could be defined as disease-modifying,” Andrew Barwicki, company spokesperson, told BioSpace.
In the Phase IIb/III trial, blarcamesine slowed clinical progression of Alzheimer’s by 38.5% at 48 weeks, compared to placebo. Analysis of the Phase IIb/III trial showed no associated neuroimaging adverse events and, unlike Kisunla and Leqembi, blarcamesine is not expected to require routine MRI monitoring.
“Blarcamesine could be appealing because of its route of administration and good comparative safety profile,” Barwicki suggested.
Anavex anticipates submitting a full regulatory package to the European Medicines Agency by the end of this year.
Novo Nordisk’s Semaglutide
Novo Nordisk is testing semaglutide, its wildly popular glucagon-like peptide-1 (GLP-1) receptor agonist for weight loss and other indications, in patients with early Alzheimer’s. Completion of the Phase III study is expected in October 2026. Early studies by independent scientists suggest semaglutide reduces neuroinflammation, which impairs cell signaling. It also may improve vascularization.
Neurim’s Piromelatine
At Neurim Pharmaceuticals, a Phase III trial in Alzheimer’s patients with mild dementia will determine whether piromelatine can improve cognition and function by improving sleep quality. The hypothesis is that sleep disturbances increase synaptic and metabolic activity, impairing the brain’s ability to clear wastes, which then increases amyloid beta aggregation. Piromelatine works by activating certain melatonin and serotonin receptor agonists.
Cassava Sciences’ Simufilam
Finally, Austin, Texas–based Cassava Sciences seeks to restore the brain’s shape and function by stabilizing the scaffolding protein filamin A (FLNA), thus purportedly blocking signaling from soluble amyloid beta.
But Cassava’s conduct around simufilam has spurred an investigation by the U.S. Department of Justice. The company’s former science advisor, Hoau-Yan Wang, was indicted in June for allegedly falsifying scientific data that led to the awards of some $16 million in NIH grants between 2015 and 2023. The charges include allegedly misconstruing data about how “a potential treatment” and its diagnostic test would work.
The company’s August corporate overview reiterates a February news release, noting that patients with mild Alzheimer’s who were treated with its oral therapeutic, simufilam, continuously for two years had no decline in their ADAS-Cog scores. Non-continuous treatment resulted in a one-point ADAS-Cog decline.
In late July, Cassava added an open-label extension to its ongoing Phase II and III clinical trials for Alzheimer’s, which attracted 89% of its Phase III patients.
According to August’s corporate overview, Cassava expects topline results from its 804-patient RETHINK-ALZ trial by year’s end.
Combinations Are Key
Alzheimer’s is a very hetergeneous disease and there is no magic bullet for treatment. Given the many different mechanisms of action of emerging Alzheimer’s therapeutics, “there may be a case for teaming up and using certain therapeutics in combination,” Tsai said.
“We’re excited to see the clinical trial pipeline diversity over time,” Edelmayer added, but the solution may involve more than therapeutics. Ultimately, “Treatment may require a combination of powerful, FDA-approved therapies in conjunction with brain-healthy lifestyles. We envision a future where there are many treatments that address the disease in multiple ways.”
https://www.biospace.com/drug-development/late-stage-alzheimers-pipeline-goes-beyond-amyloid-and-tau