Johnson & Johnson is hoping to expand the use of its prostate cancer drug Erleada (apalutamide) in patients with metastatic castration-sensitive prostate cancer (mCSPC) – and new data presented at ASCO sheds light on the dossier under review at the FDA for this early stage disease.
Developed by J&J’s pharma unit Janssen, Erleada is already approved in the big battle ground in prostate cancer – castration resistant metastatic disease – where its big rival is Pfizer/Astellas’ Xtandi (enzalutamide) and generics of its older drug Zytiga (abiraterone).
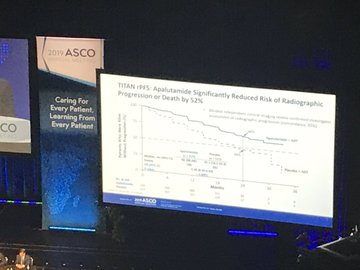
Data published at ASCO, and simultaneously in the New England Journal of Medicine (NEJM) showed adding Erleada to androgen deprivation therapy (ADT), compared with placebo plus ADT, significantly improved the dual primary endpoints of overall survival (OS) and radiographic progression-free survival (rPFS) in mCSPC.
The study included patients with mCSPC regardless of extent of disease or prior docetaxel treatment history.
Data show Erleada plus ADT significantly extended OS compared with placebo plus ADT, with a 33% reduction in risk of death.
It also significantly improved rPFS compared to placebo plus ADT with a 52% reduction in risk of radiographic progression or death compared to placebo plus ADT.
The two-year OS rates, after a median follow-up of 22.7 months, were 82% for Erleada plus ADT compared to 74% for placebo plus ADT.
A secondary endpoint of prolonged time to cytotoxic chemotherapy in patients treated with Erleada plus ADT was also met, with a 61% risk reduction compared with placebo.
In exploratory endpoints, median time to PSA progression was more favourable following Erleada plus ADT, compared with placebo plus ADT, and prostate-specific antigen (PSA) reached undetectable levels in 68% of patients in the Erleada plus ADT arm and 29% of patients in the placebo plus ADT arm.
Erleada plus ADT, compared with placebo plus ADT, achieved a 34% risk reduction in median time to second progression-free survival (PFS2), defined as time from randomisation to either disease progression on first subsequent anticancer therapy or death, whichever occurred first.
The biggest issue safety-wise was a rash, seen in 27% of patients although investigator Dr Kim Chi said most cases were Grade 1 or 2. Overall the safety profile was consistent with previous experience of the drug, he noted.
It’s bad form to pre-judge whether the FDA will approve or not, but the response from oncologists gathered at ASCO seemed to be favourable.
And while the data has gone down well in Chicago, there are also several other phase 3 studies involving rival therapies such as Bayer’s darolutamide, Pfizer/Astellas’ Xtandi, and Takeda’s orteronel – which could provide competition even if the FDA does grant a new indication.