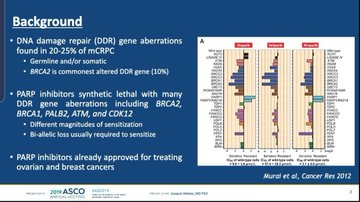
PARP, or poly (ADP-ribose) polymerase, inhibitors may have an amusing-sounding name but an increasing body of evidence suggest they could be used in a wide range of cancers as long as certain mutations are present.
PARP drugs work by interfering with cancer cells’ DNA repair system, eventually causing them to self-destruct – and AstraZeneca and Merck & Co’s Lynparza (olaparib) was the trailblazer in this drug class after first approval in ovarian cancer in 2014.
But since then the drug has gone on to be approved in breast cancer, and it looks possible that prostate cancer could be another use.
Clovis Oncology already unveiled data from the TRITON2 study at the European Society for Medical Oncology (ESMO) conference in the autumn that suggest its drug Rubraca (rucaparib) could be an option in this disease.
And the latest data from AZ and Merck & Co suggest olaparib could be a potential competitor in metastatic castration resistant prostate cancer (MCRPC) with DNA damage repair mutations.
This was a mid-stage trial that was expanded from an initial phase 1 trial to determine dose – and the study’s designers opted to include a patient arm treated with a lower 300ml dose of the drug, as well as the 400ml dose.
This was because of a regulatory update as olaparib was approved at the lower dose in ovarian cancer after it was first launched.
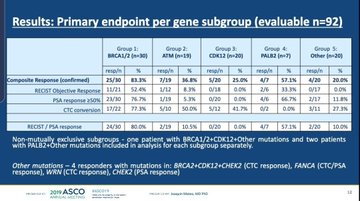
The strongest response rate from olaparib in TOPARP-B (54%) was seen in patients treated with a higher dose (400ml twice daily), although those on the more recently approved 300ml regime also benefited with a response rate of 39%.
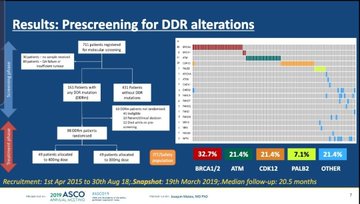
But the results also suggested that response changed according to sub-groups of patients with different DNA damage repair mutations.
Expert Mary-Ellen Taplin, of the Dana Farber Cancer Institute, praised the design of the study in an analysis and was supportive of olaparib’s potential in this new indication.
But she did raise an issue about how this drug could be used in a real-life setting: selecting patients with the mutations that are most likely to respond could be tricky.
“The experience provided warns that identifying appropriate patients within routine clinical practice will be challenging and will require repeated testing,” she said.
This could prove a difficult technical challenge – but the data were well received by clinicians gathered at the conference in Chicago.


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.