A high-protein diet, often recommended as a way to lose weight and stay healthy, appears to be harmful to the kidneys in individuals with apparently normal kidney function, two separate new studies indicate.
The two studies, from the Netherlands and Korea, were published online in Nephrology Dialysis Transplantation.
Many previous studies have shown that a high-protein diet may harm kidney function, and this is why nephrologists recommend patients with known early stage
chronic kidney disease (CKD) stick to a low-protein diet.
But people who have mild CKD of which they are unaware or those at high risk may follow the trend of eating a protein-rich diet because they believe it is healthy, say Kamyar Kalantar-Zadeh, MD, PhD, and colleagues in an
accompanying editorial.
“The high-protein culture has emerged as the preferred, healthy, and safe way of eating at the dawn of the 21st century,” they write.
Dietary regimens such as the Atkins, Zone, South Beach, and Ketogenic diets have emerged “in which daily protein intake increased to 20% to 25% or more of the total daily energy intake. We are being told that getting plenty of protein is the revival of our hunter–gatherer ancestral spirit and it will help maintain our lean muscle and reduce fat mass,” the editorialists note.
But given these two new studies, “and other data, it is time to unleash the taboo and make it loud and clear that a high-protein diet is not as safe as claimed, as it may compromise kidney health and result in a more rapid kidney function decline in individuals or populations at high risk of CKD,” they underscore.
“It is prudent to avoid recommending high-protein intake for weight loss in
obese or diabetic patients, or those with prior cardiovascular events, or a solitary kidney if kidney health cannot be adequately protected,” they summarize.
“It is essential that people know there is another side to high-protein diets and that incipient kidney disease should always be excluded before one changes one’s eating habits and adopts a high-protein diet,” added the senior author of the editorial, Denis Fouque, MD, PhD, of Centre Hospitalier Lyon-Sud, France, in a press release issued by the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA).
Dutch Study: Protein Intake, CKD Risk Highest in Those With Diabetes
In the
Dutch study, Kevin Esmeijer, MD, of Leiden University Medical Center, the Netherlands, and colleagues collected dietary data using a food frequency questionnaire from 4837 patients 60-80 years of age with a history of
myocardial infarction involved in the Alpha Omega Trial.
“At baseline and 41 months follow-up, serum cystatin C (cysC) and serum
creatinine were measured from stored blood samples,” the investigators explain.
The mean age of the cohort was 69 years and mean estimated glomerular filtration rate (eGFR) was 82 mL/min/1.73m2. As the authors point out, compared with the general population, patients with a history of myocardial infarction have double the rate of annual decline in kidney function and thus are at higher risk for CKD.
For the entire cohort, mean total protein intake was 71 g/day, of which approximately two thirds was from animal protein and the remaining third from plants.
Analyses indicated that the total amount of protein intake per day was inversely associated with the annual rate of kidney function decline. The annual change in eGFRcysC was doubled in patients with a total daily protein intake in excess of 1.20 g/kg ideal body weight, compared with an intake less than 0.80 g/kg.
Specifically, the annual change in eGFRcysC in those with the highest total daily protein intake was –1.60 mL/min/1.73m2 compared with –0.84 mL/min/1.73m2 for those with the lowest total daily protein intake, the investigators report.
And for each extra daily intake of animal protein of 0.1 g/kg ideal body weight, there was an additional decline in eGFRcysC of –0.12 mL/min/1.73m2 per year, they point out.
Subgroup analyses also indicated that the association between protein intake and decline in eGFR was threefold stronger in patients with diabetes compared to those without diabetes.
“Despite the fact that our patients received state-of-the-art drug treatment, we observed a beneficial effect of a low-protein intake on kidney function,” the authors conclude.
Higher Protein Intake and Independent Risk for Renal Hyperfiltration
In the
Korean study, Jong Hyun Jhee, MD, of the Institute of Kidney Disease Research, Yonsei University, Seoul, and colleagues analyzed the effect that a high-protein diet had on renal hyperfiltration and declining kidney function in 9226 participants from the Korean Genome and Epidemiology Study.
Patients were classified into quartiles of daily protein intake as assessed by a food frequency questionnaire. The mean age of study participants was 52 years and the mean follow-up was 11.5 years.
Among the four quartiles of daily protein intake, the prevalence of renal hyperfiltration (defined as an eGFR with residuals > 95th percentile after adjustment for confounders) was significantly higher among those in the highest quartile of protein intake, at 6%, compared with 5.2% among those in the lowest protein intake quartile (P = .02), the investigators report.
And the annual mean decline in eGFR was again highest, at –2.34 mL/min/1.73m2, among those in the highest quartile of daily protein intake, compared with –2.01 mL/min/1.73m2 among those in the lowest quartile of protein intake (P = .02).
The researchers also looked at whether a higher protein intake was associated with a greater risk for a rapid decline in kidney function, defined as a decrease in eGFR of > 3 mL/min/1.73m2 per year.
They found that those in the highest quartile of protein intake had a 32% greater risk of experiencing a rapid decline of eGFR per year compared with those in the lowest quartile (P = .03).
Jhee and colleagues then took further steps to substantiate their findings. First, they divided the cohort into those with and without renal hyperfiltration and observed that the mean daily protein intake was higher in participants who had renal hyperfiltration compared with those who did not (P = .02).
They then found that the faster drop in renal function happened only among those with preexisting hyperfiltration.
“These findings lead us to infer that a higher intake of protein may be an independent risk factor for renal hyperfiltration that can accelerate deterioration of kidney function,” they conclude.
Western Societies Consume Too Much Protein
In their editorial, Kalantar-Zadeh and colleagues caution that the new studies “should be qualified for their epidemiologic nature, given that the association does not equate to causality.” And the use of a food frequency questionnaire in both studies “is another limitation,” they observe. Furthermore, “glomerular hyperfiltration cannot be reliably detected by eGFR values.”
Notwithstanding these limitations, the studies suggest that high daily protein intake may have deleterious effects on kidney health in the general population, they state.
The recommended dietary allowance for protein intake is only 0.8 g/kg/day and the requirement for protein is likely even lower, at only about 0.6 g/kg/day, provided adequate essential amino acids are consumed, they explain.
“However, most adults in Western societies eat 1.0 to 1.4 g/kg/day of protein,” the editorialists note, “[and] protein intake may be as high as 20% to 25% or more of the total energy source,” they add — considerably higher than the 10% to 15% recommended by most guidelines.
“Emerging data across individuals and populations suggest that glomerular hyperfiltration associated with a high-protein diet may lead to a higher risk of de novo CKD or may accelerate progression of preexisting CKD,” the editorialists conclude.




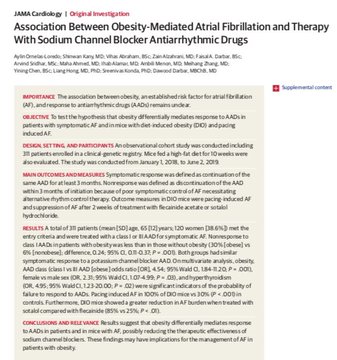
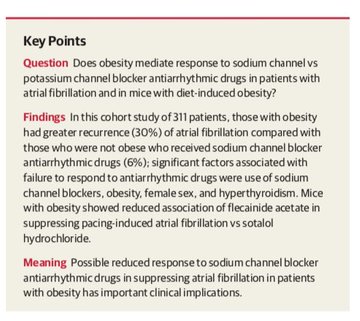
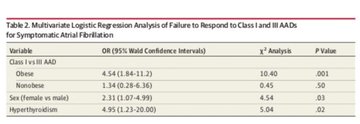
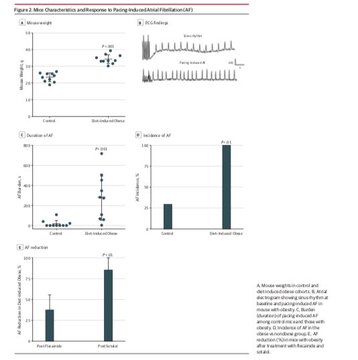
 making sodium channel blockers less helpful & possibly harmful for symptomatic control
making sodium channel blockers less helpful & possibly harmful for symptomatic control