Axovant was a big success at its IPO due to the significant potential of its investigational drugs. Nevertheless, the clinical binaries were not in the company’s favor.
Intepirdine was unable to post the strong (phase II and III) data in various franchises.
Nelotanserin also delivered subpar results in its phase II clinical program for Lewy Body dementia with visual hallucination.
There is still a chance that nelotanserin can deliver positive outcomes for the other trial studying Lewy body dementia with REM behavior disorder.
Introduction
Over 50 million individuals across the globe suffer from dementia. Dementia, which is a broad classification of many neurological diseases affecting memory and cognitive abilities, greatly hinders the afflicted patients from living a normal daily life and is one of the leading causes of death. Some of the well-known typegs of dementia are Alzheimer’s disease, Lewy Body dementia, and Parkinson’s disease. There is still no cure available despite ongoing research efforts in both academia and the pharmaceutical industry.
The drugs which were approved for Alzheimer’s disease – cholinesterase inhibitors and an NMDA antagonist – have limited therapeutic benefit or cause serious adverse events. There is an urgent need for safe and efficacious treatments for dementia. Axovant Sciences (NASDAQ:AXON) is a pharmaceutical startup focused on developing small molecule therapeutics to treat dementia and related neurodegenerative diseases. The company was founded in 2014 as a subsidiary of Roivant Sciences, both of which were founded and managed by Vivek Ramaswamy.
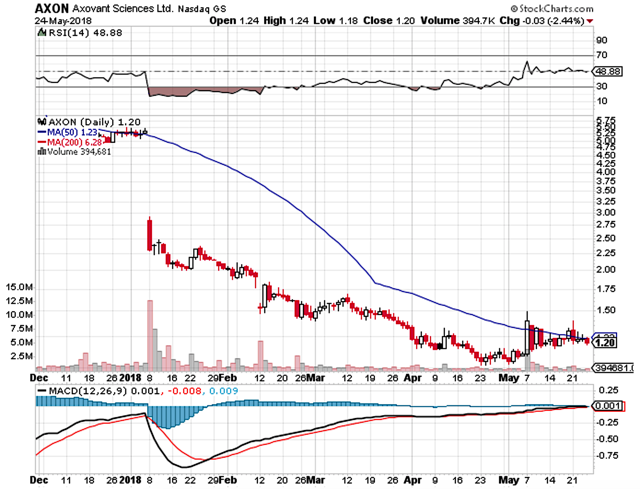
Figure 1: Axovant stock chart (Source: StockCharts)
About The Company
Axovant’s commercialization pipeline introduced two therapeutic drugs, intepirdine and nelotanserin, for a variety of clinical indications (see Table 1). Both of these investigational drugs were initially developed by other pharmaceutical companies and later acquired by Axovant.
| Drug | Clinical Indication | Status | Date |
| Intepirdine | Alzheimer’s disease | Phase III primary endpoints missed | September 2017 |
| Dementia with Lewy Bodies | Phase II negative data | January 2018 | |
| Gait and balance impairments in dementia | Phase II negative data | January 2018 | |
| Nelotanserin | Visual hallucinations in patients with Lewy Body dementia | Phase II dementia endpoint missed | January 2018 |
| Lewy Body dementia patients with REM Behavior Disorder | Phase II results pending | 3rd quarter 2018 |
Table 1. Therapeutic Pipeline (Source: data compiled from BioPharmCatalyst)
Intepirdine
Intepirdine is an antagonist for the 5-HT6 receptor, a subtype of serotonin receptors; preclinical studies suggest that the blockage of 5-HT6 receptors enhances cognitive performance and memory. An antagonist binds to a receptor and blocks its activation. The 5-HT6 receptor is a GPCR which binds to serotonin and effectuates excitatory neurotransmission. The 5-HT6 receptor is exclusively expressed in the central nervous system and has a key role in modulating cognitive processes. The absence of the 5-HT6 receptor elsewhere makes it an attractive target for treating dementia with reduced side effects. Intepirdine, at the time known as SB-742457, was initially developed by GlaxoSmithKline (NYSE:GSK) for the treatment of Alzheimer’s disease and demonstrated safety and tolerance through phase II clinical trials. Axovant acquired intepirdine, now also known as RVT-101, in December 2014 and pursued investigations for its therapeutic efficacy in several clinical indications.
MINDSET phase III study: In 2015, Axovant sought to examine whether intepirdine could provide a therapeutic benefit as an adjunctive therapy to Donepezil treatment in patients with mild-to-moderate Alzheimer’s disease. This was a double-blind, randomized study comparing daily dose of 35 mg intepirdine versus equivalent placebo in addition to 5 mg or 10 mg Donepezil through a 6-month treatment course. The aim of MINDSET was to confirm the results from the previous phase II study by GlaxoSmithKline and meet two primary outcome measures: (1) Change from baseline on the Alzheimer’s Disease Assessment Scale – Cognitive Subscale (ADAS-Cog), and (2) Change from baseline on the Alzheimer’s Disease Cooperative Study – Activities of Daily Living (ADCS-ADL) scale. The ADAS-Cog evaluates language and memory through tests like recalling words from a list or following a series of commands; the ADCS-ADL assesses the level of disability or need for assistance through examining capacity for activities ranging from dressing to shopping. The top line results were released by Axovant in September 2017 and were disappointing; although daily doses of 35 mg intepirdine was well-tolerated, there was no significant improvement in cognition or in activities of daily living.
HEADWAY-DLB & gait and balance phase II studies: In 2016, Axovant initiatedtwo more investigations with intepirdine, one of which was for treatment of dementia with Lewy Bodies. It was a double-blind, randomized study comparing daily dose of either 35 mg or 70 mg intepirdine versus equivalent placebo through a 24-week treatment course. The primary outcome measure was a change from baseline on Unified Parkinson’s Disease Rating Scale (UPDRS) part III. Part III is the examination of motor function, which includes assessing speech, facial recognition, tremor at rest, etc. In parallel, Axovant pursued investigation of using intepirdine to aid gait and balance in patients afflicted with any dementia, whether Alzheimer’s disease, dementia with Lewy Bodies, or Parkinson’s disease. This was a double-blind, randomized study in which patients were enrolled in one of two sequences: daily dose of 35 mg intepirdine during early treatment period followed by placebo during the late treatment period, or vice-versa. The primary outcome measure of this crossover assignment study was a change in quantitative gait measurements from baseline to the end of the 12-week treatment. In January 2018, negative results from both the HEADWAY and the gait and balance phase II studies were posted. At this point, intepirdine had failed for all primary endpoints in every clinical trial. Despite intepirdine being well-tolerated, it was not efficacious and was ultimately pulled from any further investigational efforts.
Nelotanserin
Nelotanserin is an inverse agonist for the 5-HT2A receptor, a subtype of serotonin receptors. An agonist binds to a receptor and induces a biological effect. An inverse agonist binds to the same receptor as an agonist but induces the opposite biological effect. In rats, nelotanserin was found to enhance slow wave sleep and reduce the frequency of awakening. At the time named APD-125, nelotanserin was under development with Arena Pharmaceuticals (NASDAQ:ARNA) for the treatment of insomnia. Clinical trials demonstratedthe effectiveness and safety of nelotanserin for treating insomnia, but its development was stopped because it did not meet the primary endpoints. In 2015, Axovant acquired nelotanserin and began pursuing two clinical trials for treating visual hallucinations and sleep disorders in patients with Lewy Body dementia.
Visual hallucination phase II: Axovant initiated a study to examine whether nelotanserin could be effective for treating visual hallucination for patients with Lewy Body dementia. This was a double-blind, randomized study in which subjects were enrolled in one of two sequences: daily dose of 40 mg nelotanserin for two weeks followed by 80 mg nelotanserin for two weeks during the first treatment period followed by placebo during the second treatment period, or vice-versa. There were two primary outcome measures: (1) assessing safety based on incidence of adverse events and significant changes, from baseline to the end of treatment period, in physical examinations, vitals, and other routine clinical evaluations, and (2) evaluation of extrapyramidal signs via UPDRS parts II and III. Part II assesses the ability to perform activities of daily life through speech, salivation, swallowing, etc. Part III was described earlier in the HEADWAY section. In January 2018, Axovant announced results indicating that nelotanserin was well-tolerated and demonstrated a positive trend in efficacy. However, the next day, Axovant announced a correction: the p value announced was incorrect (initially declared as p=0.011, but was actually p=0.531) and the efficacy endpoint was missed.
REM sleep behavior disorder phase II: Axovant sought to examine whether nelotanserin could be effective for treating REM sleep behavior disorder in patients with dementia. This was a double-blind, randomized study in which patients would receive either daily dose 80 mg nelotanserin or equivalent placebo for 28 days. Recruited patients had either dementia with Lewy Bodies or Parkinson’s disease. The primary outcome measure was the change in frequency of REM sleep behaviors from baseline to the end of the treatment course. This study is still underway, and results are due in the third quarter of 2018.
Financials Assessment
As depicted in Figure 2, there were no revenues for the trailing twelve-month (“TTM”) revenues. In addition, there was a net loss of $242.37 million (-$2.36 per share) for the TTM, compared to $180.9 million (-$1.82 per share) declines for fiscal 2017. Investors should be cognizant that it is the norm for a relatively young bioscience like Axovant to incur significant losses for many years prior to banking a net profit (due to the lengthy and low success rate of the innovation process). Nonetheless, it only takes one blockbuster to make your investment worthwhile. In viewing the balance sheet, there were $188.2 million in cash as of December 2017, versus $212.5 million. Based on these metrics, the company may raise capital via a public offering in the foreseeable future.
Figure 2. Key financial metrics (Source: Morningstar)
Further Discussion
Axovant Sciences held great promise and was eager to sell itself as the company with solutions for dementia. The company raised $315 million during its initial public offering (IPO) in 2015 and ended its first trading day with the stock price nearly doubled (from $15.00 to $29.90). Shareholders were extremely excited, and at the time it was the largest IPO in the biopharmaceuticals industry. However, after a series of failed clinical trials, shareholders lost confidence, and the stock now trades well below $2.00 (as of March 2018).
Potential Risks
For a small bioscience firm, the primary risk is whether the lead molecule will pass the clinical trial. If the drug fails to post positive data, the stock can tumble over 50%. Conversely, if the data report is positive, investors can expect the stock to catapult to the new high by a similar (or greater) magnitude. With that being said, the main concern for Axovant at this point is if nelotanserin can post the positive data for the phase II study (Lewy Body Dementia patients with REM Behavior Disorder). Give the previous failures, the risks of a negative clinical binary are significantly high. Moreover, even if the aforesaid medicine is approved, it might not generate substantial sales due to market competition and other unforeseen variables. That aside, the other concern is whether the additional cash is needed to fund innovation going forward.
Quantitative Data Forecasting (nelotanserin)
Leveraging our Integrated BioSci framework of “molecule analysis” – that took into accounts different scoring variables, including available trial data (“TDV”), comparative molecular analysis (“CMV”), structural design (“SDV”), clinical trial setups (“TSV”), and disease specificity (DSV) – we prognosticated that there are only 45% chances that nelotanserin will procure positive outcomes in the phase II trial (for Lewy Body Dementia patients with REM Behavior Disorder). Notably, the TDV variable factored substantially into this data forecast. That said, the quantitative variables scored quite unfavorably, but the qualitative metrics are more promising.
Qualitative Metrics Assessment
Due to the intellectual generosity of a member of Integrated BioSci Investing, having the experience as an oncology product strategist, we learned to implement the qualitative metrics assessment. The three variables – science novelty (underlying product differentiation), unmet medical needs (signifying therapeutic demand), and ease of regulatory approval (suggesting chances of success) – are ranked as high, medium, and low, respectively. There are also different degrees of a qualitative score for the aforementioned variables (i.e., extremely high or high). Of note, these factors are best used in combinations with the quantitative metrics in the data forecasting section. Per Table 2, two of the three qualitative variables (barring science novelty) for nelotanserin ranked high, thus indicating a favorable regulatory outcome (that is, if and only if the clinical data will be positive).
| Qualitative Data Analysis (nelotanserin) | |
| Science novelty (product differentiation) | Medium |
| Unmet medical needs (therapeutic demand) | High |
| Ease of regulatory approval | High |
Table 2: Qualitative metrics assessment (Source: Dr. Tran BioSci)
Conclusions
In all, we take a pass on Axovant with two out of five stars rating. There were several red flags throughout the investigations pursued by the company. First, Axovant was led by a very young (29 years old at the time) hedge fund manager with limited experience in drug development. Second, the drugs under development by Axovant were acquired from other pharmaceutical companies which had halted their own clinical trials despite the significant time and resources used. Although these drugs had supportive preclinical data and passed initial clinical trials, caution was warranted as the mid-stage clinical trials failed. It was great that tolerance and safety was achieved, but ultimately, efficacy is the requirement for an investigational drug to become the standard of practice. Third, Axovant made a very significant mistake in reporting the results for the visual hallucination phase II clinical trial, either due to poor management or, seemingly, to mislead shareholders.
Although the investigation of intepirdine was halted, there is a small chance that nelotanserin can still offer a potential solution for patients with dementia. Axovant recently made significant changes in its management team and board of directors, with hopes of solidifying its efforts on nelotanserin and rebuilding the company’s product portfolio. However, this may be a big lesson for the venture capital and investment community that money alone doesn’t buy efficacious, blockbuster drugs.

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.