The SanBio Group, a scientific leader in regenerative medicine for neurological disorders, today announced the acquisition of a patent portfolio regarding cell medicines derived from mesenchymal stem cells (hereinafter the Patent Portfolio).
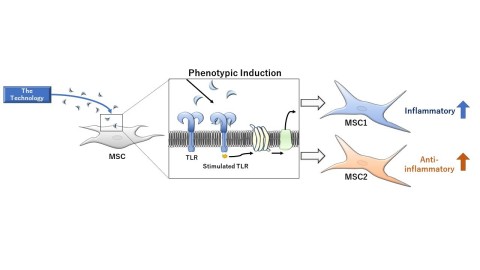 Image of the Technology of MSC1 and MSC2 (Graphic: Business Wire)
Image of the Technology of MSC1 and MSC2 (Graphic: Business Wire)
The Patent Portfolio is pertaining to technology (hereinafter the Technology) discovered by Dr. Aline Betancourt, a researcher at Tulane University, that enhances anti-inflammatory and pro-inflammatory functions of mesenchymal stem cells (MSCs)*1 by stimulating specific Toll-like receptors (TLRs)*2expressed on mesenchymal stem cell membranes while maintaining safety and tolerability profile of mesenchymal stem cells. Mesenchymal stem cells induced to have enhanced anti-inflammatory function (MSC2 phenotype) and those induced to have reinforced pro-inflammatory function (MSC1 phenotype) using the Technology are more likely to migrate to injury sites and express higher homogeneity compared with naïve, uninduced mesenchymal stem cells, indicating their therapeutic potential as highly safe and effective agents.
The MSC2 phenotype, with its enhanced anti-inflammatory function, has therapeutic potential against demyelinating disorders (e.g., optic neuritis, multiple sclerosis, and Krabbe disease), diabetic neuropathy, and inflammatory disorders (e.g., rheumatoid arthritis and Crohn’s disease). Pre-clinical studies using animal models of each disease are currently underway. Regarding the MSC1 phenotype, which demonstrates reinforced pro-inflammatory function, pre-clinical studies have shown that it attenuates tumor growth as opposed to uninduced mesenchymal stem cells that promote proliferation of tumor cells. Based on such findings, development of MSC1 phenotype for the treatment of cancer is highly anticipated.
SanBio is currently conducting phase 2 clinical trials of regenerative cell medicine SB623 in its propriety development pipeline targeting chronic motor deficit from ischemic stroke in the US and targeting chronic motor deficit from traumatic brain injury (TBI) in Japan. SB623 consists of mesenchymal stem cells derived from adult bone marrow that undergo a genetic modification. It is a stem cell-based therapy with potential to promote brain tissue regeneration that helps patients with ischemic stroke or TBI recover lost functions when administered to the area around the injury site. In animal studies where SB623 was administered to brains of mouse models of ischemic stroke, phenomena such as migration of neural stem cells to injury sites, proliferation of nerve cells, and angiogenesis were observed.
Acquisition of the Patent Portfolio will enable SanBio to expand its development pipeline into areas of inflammatory disorders and cancer in addition to neurological disorders targeted by SB623. By leveraging its knowledge and expertise relating to mesenchymal stem cells SanBio has cultivated so far, SanBio will work to develop new cell medicines based on the Patent Portfolio.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.