Dr. Fred Saad gave a presentation on an exploratory analysis using the DECIPHER test in patients included in the SPARTAN trial.
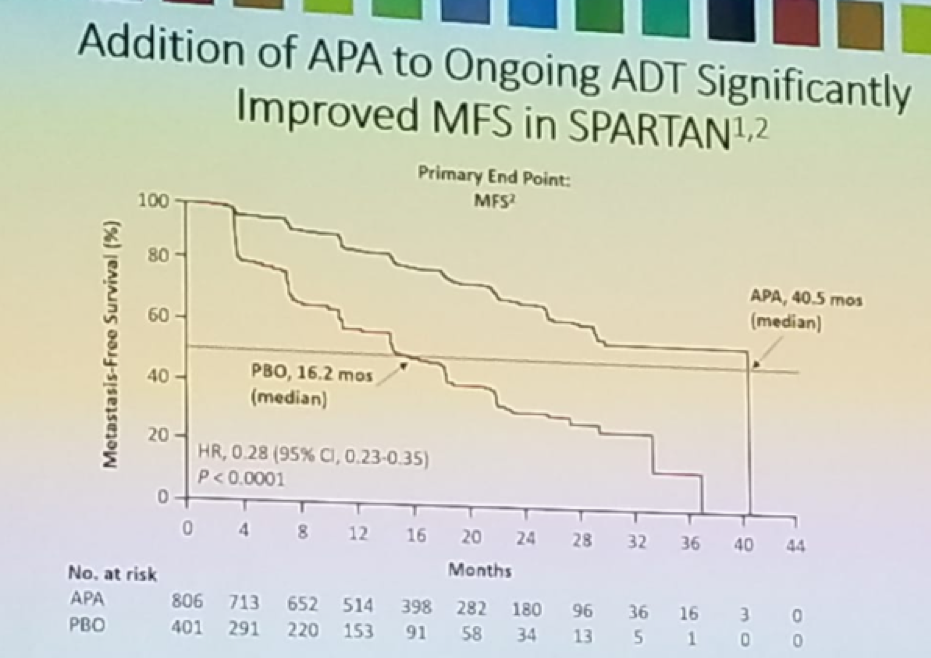
Patients with non-metastatic castrate-resistant prostate cancer (nmCRPC) and a PSA doubling time (PSADT)<=10 months are at higher risk for metastases/death when compared to patients with longer PSADT. Androgen receptor inhibitors, including Apalutamide, enzalutamide, or Darolutamide added to ongoing androgen deprivation therapy (ADT) have been shown to improve outcomes in high-risk nmCRPC patients. In the SPARTAN trial,1 nmCRPC patients with a PSA doubling time<10 months, who were receiving ADT, were given Apalutamide and were compared to patients who were given ADT alone (figure 1). Apalutamide was shown to improve metastasis-free survival (MFS) and other secondary end points, as seen in figure 2.
Figure 1- The SPARTAN trial:

Figure 2- Apalutamide shown improve MFS in the SPARTAN trial:
The DECIPHER prostate test (GenomeDx Biosciences, Inc., San Diego, CA) is a clinical-grade 22-marker mRNA-based genomic classifier (GC) that can support treatment decisions in patients with localized disease and predict disease progression in newly diagnosed patients (2). Patients with high GC scores have a significantly higher risk for metastasis than patients with low to average GC scores.
According to Dr. Saad, in this study, the authors evaluated the association between DECIPHER GC score and MFS following Apalutamide treatment in the SPARTAN trial. This was an exploratory analysis of SPARTAN patients (figure 3). Analyzed samples were stratified into high-risk (GC score>0.6) and low-to-moderate risk (GC score <=0.6) subtypes. Approximately 50% of patients had high risk, and 50% had low to average risk DECIPHER GC score. The association between DECIPHER GC subtypes and MFS was assessed as well.
Figure 3 – Exploratory analysis in SPARTAN trial:
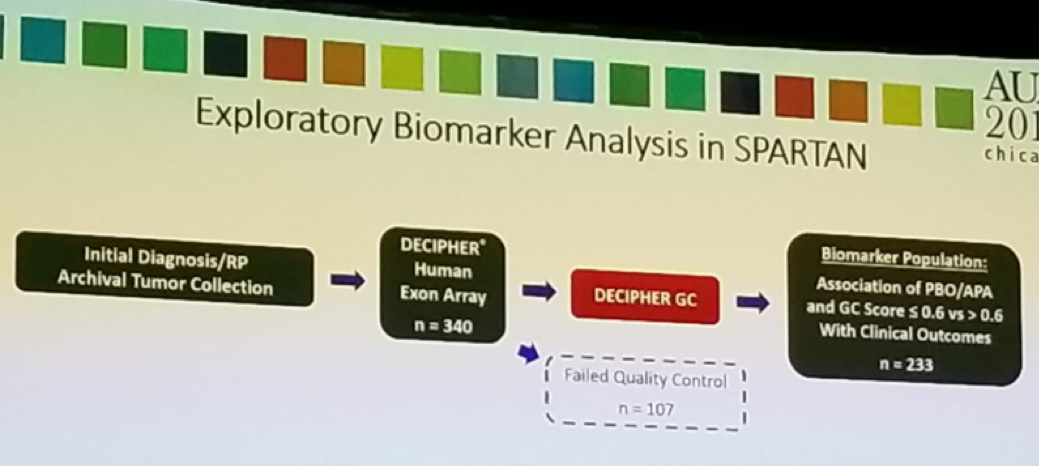
Figure 4 and figure 5 demonstrates the MFS by SPARTAN treatment arms in patients with high and low to average DECIPHER GC scores, and the MFS by DECIPHER GC score in the treatment arms, respectively.
Figure 4 – MFS by SPARTAN treatment arms in patients with high and low to average DECIPHER GC score:
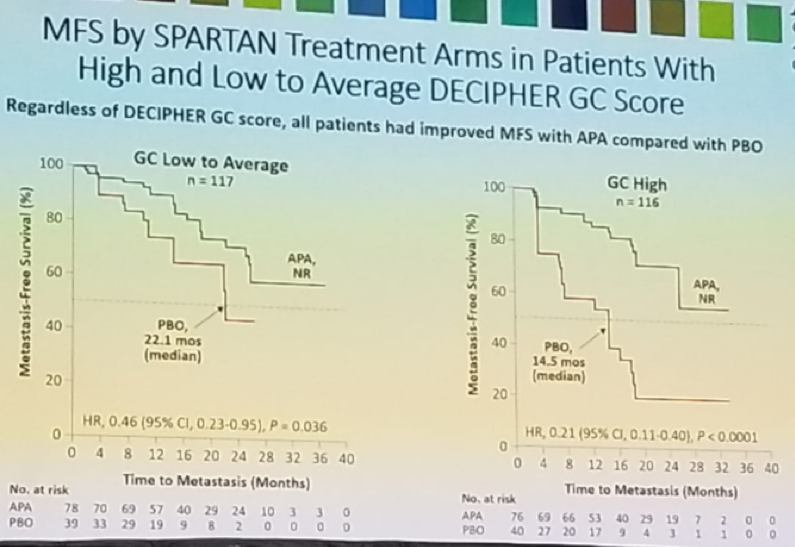
Figure 5 – MFS by DECIPHER GC score in SPARTAN treatment arms:

Dr. Saad concluded his presentation and stated that the results demonstrate, that regardless of DECIPHER GC scores, all patients had significantly prolonged MFS following the addition of Apalutamide to ongoing ADT. nmCRPC patients with high GC score are the highest risk of early metastases with ADT alone. Despite the high risk of early progression in patients with high GC score, the addition of Apalutamide to ADT was as effective in this subgroup as in the low to average GC score subgroup. Early treatment intensification with Apalutamide in the high-risk GC subgroup significantly prolonged MFS and seems to fulfill an unmet need.
Presented by: Fred Saad, MD FRCS, Professor and Chief of Urologie; Director of GU Oncology; Raymond Garneau Chair in Prostate Cancer; University of Montreal Hospital Centre (CHUM); Director, Prostate Cancer Research; Institut du cancer de Montréal/CRCHUM.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.