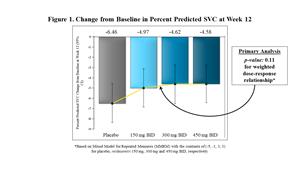
Trial Did Not Meet Statistical Significance for Primary Efficacy Analysis
| ||||||||||||||||||||
Patients on All Doses of Reldesemtiv Declined Less Than Patients on Placebo
for SVC and ALSFRS-R, With Clinically Meaningful Differences Emerging Over Time
for SVC and ALSFRS-R, With Clinically Meaningful Differences Emerging Over Time
Early Terminations and Serious Adverse Events
Were Balanced Across Treatment Arms
Were Balanced Across Treatment Arms
Investor Event & Conference Call on May 6, 2019 at 7:30 a.m. Eastern Time
Conference Call/Webcast
Cytokinetics will host an investor event and conference call on May 6, 2019 at 7:30 a.m. Eastern Time including members of management and members of the Steering Committee of FORTITUDE-ALS. The event will be held at the Loews Philadelphia Hotelin the Lescaze Room. The conference call will be simultaneously webcast and will be accessible in the Investors & Media section of Cytokinetics’ website. The live audio of the conference call is also accessible via telephone to investors, members of the news media and the general public by dialing either (866) 999-2985 (CYTK) (United Statesand Canada) or (706) 679-3078 (International) and typing in the passcode 4284768. An archived replay of the webcast will be available via Cytokinetics’ website until June 3, 2019. The replay will also be available via telephone from May 6, 2019 at 11:00 a.m. Eastern Time until June 3, 2019 by dialing (855) 859-2056 (United States and Canada) or (404) 537-3406 (International) and typing in the passcode 4284768.
Cytokinetics, Incorporated (Nasdaq: CYTK, “Cytokinetics”) announced that results of FORTITUDE-ALS (Functional Outcomes in a Randomized Trial of Investigational Treatment with CK-2127107 to Understand Decline in Endpoints – in ALS) were presented today by Jeremy Shefner, M.D., Ph.D., Lead Investigator of FORTITUDE-ALS, Professor and Chair of Neurology at Barrow Neurological Institute, and Professor and Executive Chair of Neurology at the University of Arizona, Phoenix, at a podium presentation at the American Academy of Neurology Annual Meeting in Philadelphia.
FORTITUDE-ALS did not achieve statistical significance for a pre-specified dose-response relationship in its primary endpoint of change from baseline in slow vital capacity (SVC) after 12 weeks of dosing (p=0.11). Similar analyses of ALSFRS-R and slope of the Muscle Strength Mega-Score yielded p-values of 0.09 and 0.31, respectively. However, patients on all dose groups of reldesemtiv declined less than patients on placebo for SVC and ALSFRS-R, with larger and clinically meaningful differences emerging over time.
While the dose-response analyses for the primary and secondary endpoints did not achieve statistical significance at the level of 0.05, in a post-hoc analysis pooling the doses together, patients who received reldesemtiv in FORTITUDE-ALS declined less than patients who received placebo. The trial showed effects favoring reldesemtivacross dose levels and timepoints with clinically meaningful magnitudes of effect observed at 12 weeks for the primary and secondary endpoints. The differences between reldesemtiv and placebo in SVC and ALSFRS-R total score observed after 12 weeks of treatment were still evident at follow-up, four weeks after the last dose of study drug.
The incidence of early treatment discontinuations, serious adverse events and clinical adverse events in FORTITUDE-ALS were similar between placebo and active treatment arms. The most common clinical adverse effects in the trial included fatigue, nausea and headache. The leading cause for early termination from FORTITUDE-ALS for patients who received placebo was progressive disease; the leading cause for early termination for patients who received reldesemtiv was a decline in cystatin C based estimated glomerular filtration rate (eGFR), a measure of renal function. Elevations in transaminases and declines in cystatin C eGFR were dose-related.
“Results from FORTITUDE-ALS are among the most impressive we have seen in a Phase 2 clinical trial in ALS,” said Dr. Shefner. “Especially noteworthy are the consistency and durability of effects observed across treatment arms on clinically meaningful endpoints.”
In collaboration with Astellas, Cytokinetics is developing reldesemtiv, a next-generation fast skeletal muscle troponin activator (FSTA), as a potential treatment for people living with debilitating diseases and conditions associated with skeletal muscle weakness and/or fatigue.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.