It looks like testing for germline (inherited) mutations is going to be increasingly important in pancreatic cancer.
As ASCO experts pointed out to me in this morning’s press briefing, the technology is available and could identify patients with the BRCA mutations who could respond to treatment with PARP inhibitors.
Testing for somatic BRCA-like mutations that arise as the disease progresses could also be feasible in the future.
an hour ago
Here’s some more from my interview with Merck & Co’s Dr Roy Baynes, who discussed the long-term findings of the KEYNOTE-001 data announced yesterday.
Findings were summarised in yesterday’s blog – but headline data was that after a median follow-up of 60.6 months – around five years – and at that point 18% of enrolees (100 people) were still alive.
Of those who had not received prior treatment, 23% were still alive after five years compared with 15.5% of those previously treated.
Dr Baynes said: “These were metastatic lung cancer patients. When I was training, the vast majority of metastatic lung cancer patients would be dead after five years.”
Before immunotherapy the survival rate was about 5%, but Dr Baynes pointed out that there has been a “five-fold improvement in outcomes.”
“Depending on the group of patients that you study the five-year survival is somewhere between 15% in the pretreated population in the salvage situation and around 25% in the front-line population.
“If you look at the PD-L1 strongly positive (a biomarker for immunotherapies) it’s almost 30% so huge change from the time when maybe one in 20 patients survived.
“Somewhere between one in five, one in four, one in three patients surviving after five years, so that’s a huge change in what the cancer patient can expect.”
2 hours ago
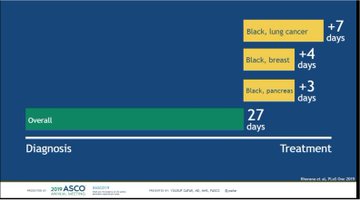
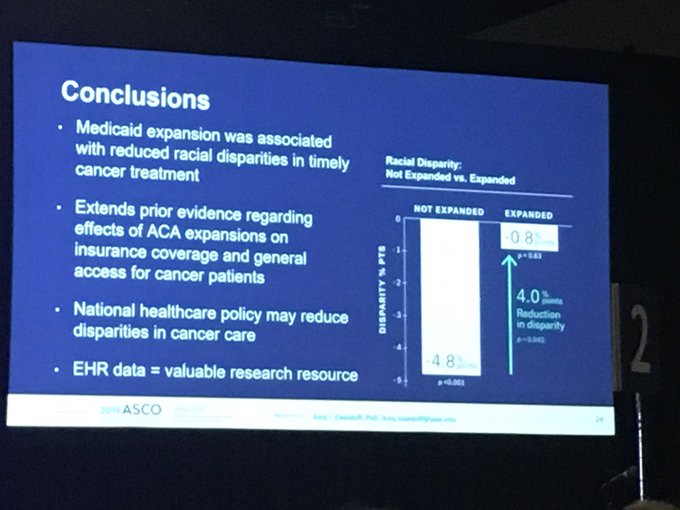
ASCO’s experts may have fudged this issue slightly this morning.
But there was plenty of reaction to the data on how Obamacare addressed racial inequality in access to cancer care when it was highlighted in a plenary session.
2 hours ago
Stressed-out cancer cells
The results in pancreatic cancer from Merck & Co/AstraZeneca’s Lynparza came from a PARP inhibitor.
The principle behind this drug is to interfere with the DNA damage repair system that certain cancer cells need to survive.
DNA damage repair is just one of several stress-relieving strategies adopted by cancer cells.
Joan Brugge director of the Harvard Ludwig Cancer Center and professor of Cell Biology at Harvard Medical School gave a fascinating talk about how drug companies could target these stress-relieving mechanisms to treat the disease.
Targeting multiple stress relieving programmes will also be highly selective for cancer cells as healthy cells don’t have these mechanisms in operation.
They could also integrate with immunotherapy for an even more powerful effect.
2 hours ago
3 hours ago
More of that data from Sanofi about isatuximab, which is being tested in combination with Celgene’s Pomalyst (pomalidomide) and dexamethasone in multiple myeloma.
Progression free survival (PFS) was 11.53 months on the isatuximab combination compared with 6.47 months on Pomalyst and dexa standard care – with a nice highly significant p-value of 0.001.
Compared to pomalidomide and dexamethasone alone, isatuximab combination therapy demonstrated a significantly greater overall response rate (60% vs. 35%) with an even better p value of 0.0001
In additional analyses, isatuximab combination therapy compared to pomalidomide and dexamethasone alone showed a consistent treatment benefit in the following subgroups: patients 75 years and older, patients with renal insufficiency, and patients who were refractory to lenalidomide. Results were confirmed by independent review committee.
Overall survival data is not ready yet by Sanofi said there is a trend towards isatuximab combination compared with standard care.
Sanofi’s combination also showed a shorter median time to first response versus pomalidomide and dexamethasone alone (35 days vs. 60 days).
3 hours ago
I just caught up with Dr Roy Baynes, chief medical officer at Merck Research Laboratories, and asked him about the data from AZ/Merck & Co’s Lynparza (olaparib) in pancreatic cancer, among other things.
The companies are developing the drug in partnership and equally share development and marketing costs, as well as profits.
While the efficacy data was strong from the POLO trial, there were some side effects with 40% of people experiencing serious side effects compared with 23% in a placebo arm.
I asked him whether this could be an issue with regulators during their reviews in this indication.
Dr Baynes said: “All drugs are going to have side effects, and if you look at discontinuation it was very uncommon. Despite an adverse event profile, much of which is on-target, anaemia, the vast majority of patients stayed on treatment.”
We also discussed the lack of overall survival data so far from POLO – this is caused by the number of patients who are still alive on this trial.
“If you look at most of the PARP inhibition data that we have generated, it really has been about progression free survival. It’s really important, it means you are not going on to some other treatment, you are not progressing and most people see this as an important clinical benefit,” he said.
He also pointed out that a small percentage of pancreatic cancer patients could be treated with Merck & Co’s Keytruda (pembrolizumab) immunotherapy if the tumour possesses “MSI-High” mutations – one of the drug’s many indications.
3 hours ago
Sanofi announced results from the first Phase 3 study of isatuximab, an investigational antibody treatment, used in combination with pomalidomide and dexamethasone, to treat people living with multiple myeloma.
It’s the second most common haematological cancer, is uncurable and characterized by multiple relapses.
The results, presented by Sanofi today showed isatuximab combination therapy prolonged the length of time a patient lived without their cancer getting worse (progression free survival) by more than five months compared to Celgene’s Pomalyst (pomalidomideP) and dexamethasone alone.
Overall response rate with isatuximab combination therapy was almost double that of the response rate seen with pomalidomide and dexamethasone alone.
4 hours ago
5 hours ago
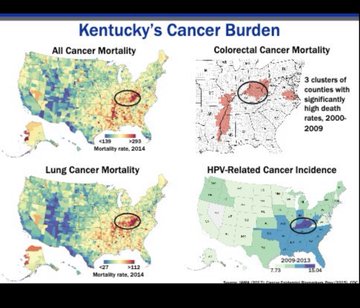
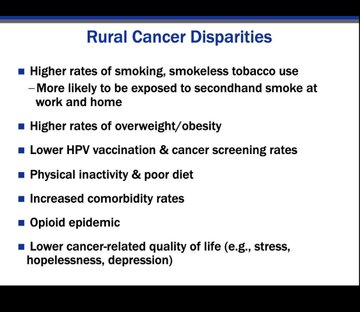
In a session on health equality Dr Robin Vanderpool highlighted some of the disparities in cancer care that affect rural communities.
These include the ‘tyranny of distance’, where cancer patients live more than 50 miles away from a hospital, and a range of socioeconomic disparities.
Dr Vanderpool was earlier this year appointed director for community outreach and engagement at the Lexington, Kentucky-based Markey Cancer Center.















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.