It’s no secret that preparation for a colonoscopy is an uncomfortable experience. At times, the preparation can be so off-putting, that some chose to forgo the procedure altogether. For this reason, Check-Cap has created an indigestible capsule for prep-free colon cancer screening to ensure that no patient gets left behind in cancer precaution. The device is already approved in Israel and Europe, and tests for U.S. approval are currently underway.
Check-Cap’s swallow-able capsule uses low-dose x-rays to screen the digestive system while it moves along the GI tract. These scans are used to primarily detect precancerous polyps in the colon, making the capsule a preventative test, not a cancer treatment.
“Most colon screening technologies use video technology while entering into the colon and this requires preparation because there can be obstructions that interfere with visualization”, explained Alex Ovadia, CEO of Check-Cap. “The creator tried to think of something that could overcome that and could change the pre-screening requirements. The technology that came to his mind was x-ray.”
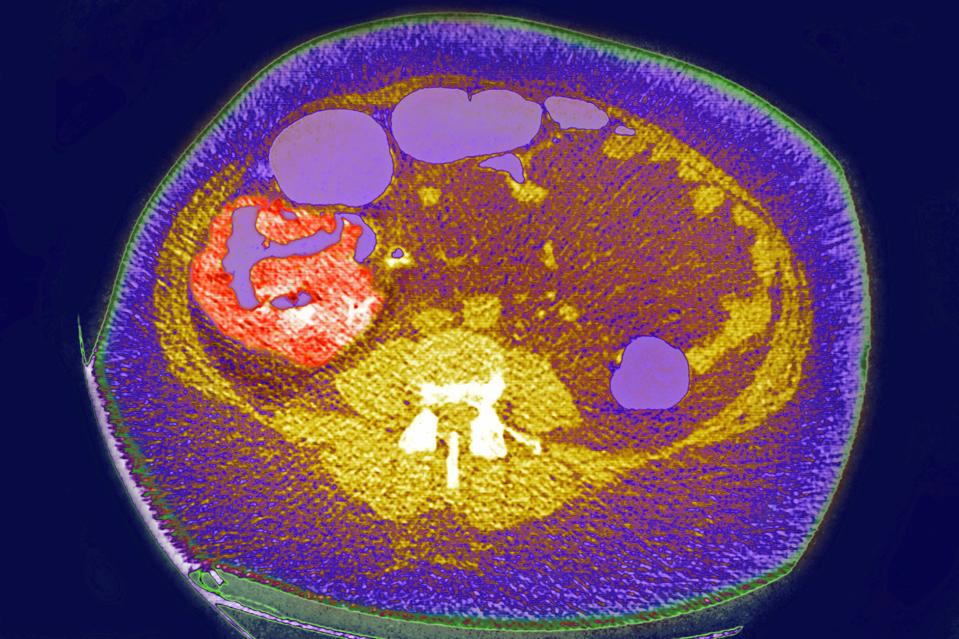
Colon cancer, tumor on the caecum and ileocolic valve, Visualization on a radial CT scan. (Photo by: … [+]
UNIVERSAL IMAGES GROUP VIA GETTY IMAGES
The test is non-invasive and patients are allowed to carry on with their normal activities as the screening is being conducted. Once the scan is completed, the capsule is eliminated through the natural excretion process and flushed away, as the data has already been transmitted to a device attached to the patient’s back. This data is then used by doctors to outline a patient’s personal cancer risk and note which patients should be advised to undergo a colonoscopy procedure.
While other devices such as the PillCam also work off swallow-able colon screening technology, the concept of using x-ray technology instead of video is unique.
X-ray technology may bring to mind dangerous and serious equipment, but according to Ovadia, the x-rays used in the Check-Cap’s capsule are low-dose and do not cause patients any harm or damage.
Colon cancer is one of the most widespread and deadly forms of cancer in the U.S. killing about 50,000 Americans annually. The gold standard for colon cancer screening is the colonoscopy, but many patients chose to forgo this in order to avoid the uncomfortable laxative cleanse required beforehand. Check-Cap cites this demographic as their target client.
“We are not trying to replace the colonoscopy,” said Ovadia. “Instead, we are aiming towards the population that is most at risk because they chose to not undergo any type of screening.”
Most colon cancer begins as growths called polyps, and it’s these pre-cancerous polyps that the capsule detects. As a result, the device aims to help patients stop cancer from forming in the first place.
“We believe that our solution is intended to prevent colon cancer ahead of time,” said Ovadia, explaining that one of the company’s slogans is “Why Wait For Cancer?”
The technology was developed in 2005 and first received the go-ahead for use in Israel, where it was originally developed. Now, Check-Cap is in the midst of undergoing the strenuous FDA qualification to allow the device to be used in the United States. Eventual U.S. commercialization is expected to begin in 2022.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.