Recently, researchers have investigated a nerve repair process that could potentially be activated to heal
injuries to the brain and spinal cord. This discovery came from investigating an existing peripheral nerve regeneration pathway and applying similar concepts to central nervous system cells. This research team was led by Claire Jacob, Professor at Johannes Gutenberg University Mainz (JGU) and at the Swiss University of Fribourg. Their findings were published in a recent edition of
Cell Reports.
Damaged peripheral nerves regenerate after an injury, allowing sensation and function to be retained. Axons, the long portions of neurons that send electrical signals, must regrow after such injuries to properly recover function.
“An injury in the peripheral nervous system quickly triggers the activation of a fascinating repair process that allows the injured nerve to regenerate and regain its function,” explains Claire Jacob, Head of Cellular Neurobiology at JGU. “There is no such repair process in the central nervous system, thus injuries often lead to permanent damage such as paraplegia.” Strategies to improve axon regeneration in the central nervous system must therefore be developed to enable healing.
Certain axons are covered in a protective, insulating layer known as myelin. This myelin sheath serves not only to protect the axon, but to increase the speed of signaling between nerves and their targets.
“Myelin is extremely important for the function of the entire nervous system, however it also hinders the repair process in case of an injury,” noted Jacob.
The myelin sheath is created by Schwann cells in the peripheral nervous system and oligodendrocytes in the central nervous system (the brain and spinal cord). One major difference between the two is that the Schwann cells have a unique repair process in response to axonal injury.
Action of the Schwann Cell
In the peripheral system, Schwann cells rapidly induce disintegration of injured axonal pieces into fragments. These fragments are ultimately digested either by Schwann cells or macrophages. Clearing these axon fragments after injury is essential in the repair process in the peripheral nervous system.
“Schwann cells can do everything,” noted Jacob. “We discovered that they not only digest myelin following injury, but they also induce the disintegration of the long axon segments that are separated from their cell bodies due to the injury.”
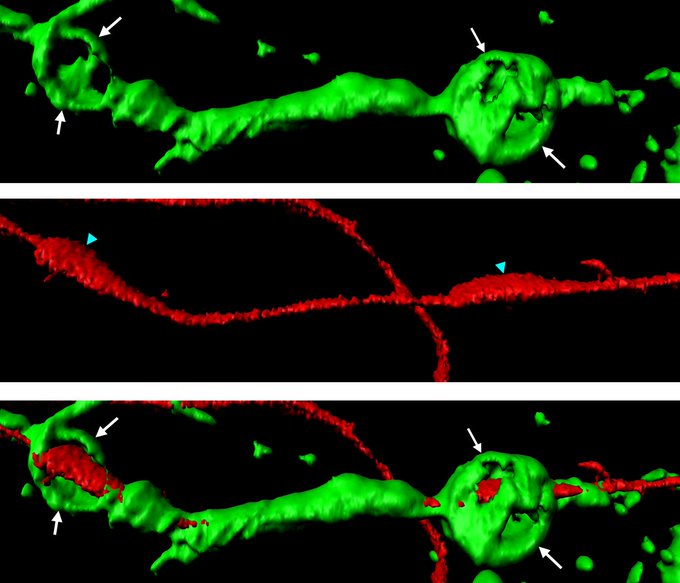
For this to occur, the Schwann cell forms small spheres of the protein actin. Actin spheres put pressure on the isolated segments of axons until they are fragmented. For the intact region of the axon to grow back, it is key that this targeted degradation of cell debris occurs. This axon must remain attached to the neuron’s cell body and regrow towards its target once more.
The researchers found that these injured axon fragments send a signal to the Schwann cells that initiates this actin sphere formation and axon disintegration. Jacob and her team noted that this process is extremely coordinated and sensitive, with disruptions causing impaired regeneration.
Action of the Oligodendrocyte
After carefully studying this process in the peripheral nervous system’s Schwann cells, the researchers shifted focus to the oligodendrocyte function in the central nervous system.
“After an injury, oligodendrocytes either die or remain apparently unresponsive,” explained Jacob.
Unlike the versatile Schwann cell, the oligodendrocyte lacks the ability to form actin spheres and disintegrate damaged axons. This is because these cells do not express vascular endothelial growth factor receptor 1 (VEGFR1) like the Schwann cells do. This receptor is needed for producing the actin spheres and is inherently crucial in axonal repair.
Jacob and colleagues decided to induce VEGFR1 expression in the oligodendrocytes to see if the cells would begin to act as the Schwann cells do. The team found that the oligodendrocytes began to produce the actin spheres and disintegrate damaged axon fragments in the same repair process as the Schwann cells.
Going Forward With this Work
They are now working to identify the biological pathways associated with myelin removal at the injury site in the central nervous system. This is the next step required for the full rebuilding of damaged neurons.
“We have discovered a pathway that accelerates myelin degradation in the peripheral nervous system and are now trying to determine whether this can also trigger myelin removal in the central nervous system,” concluded Claire Jacob.

 | First step to induce self-repair in the central nervous system. The research team led by Prof. Claire Jacob
| First step to induce self-repair in the central nervous system. The research team led by Prof. Claire Jacob