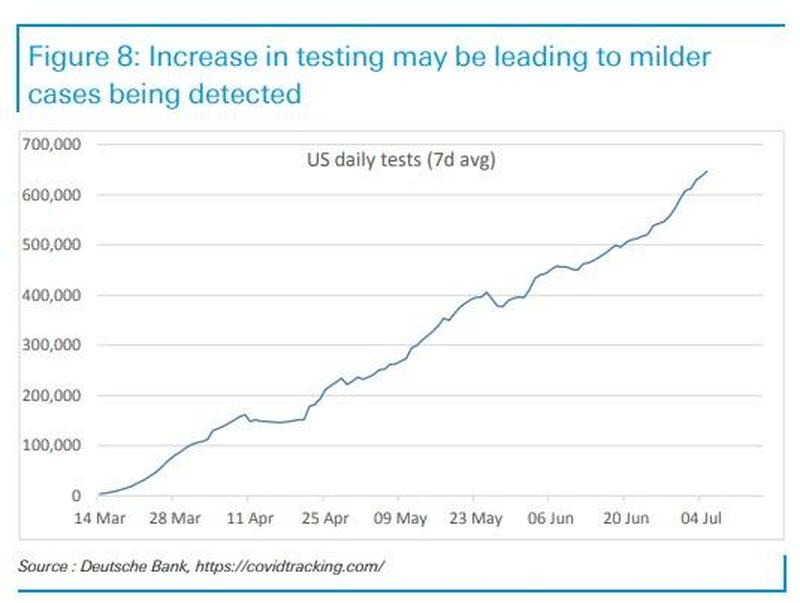
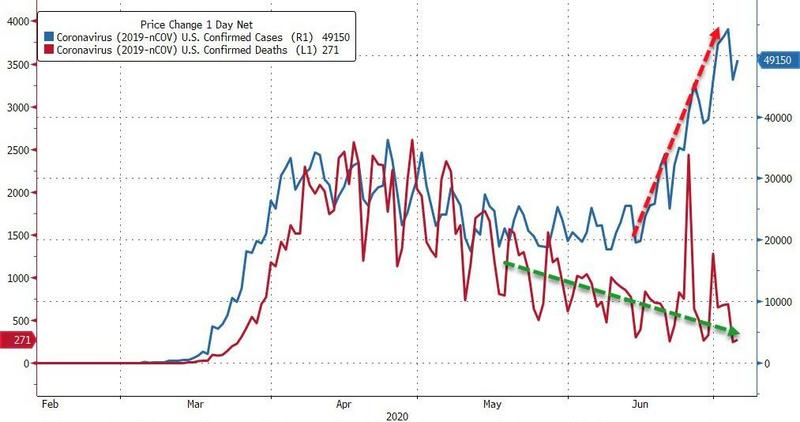
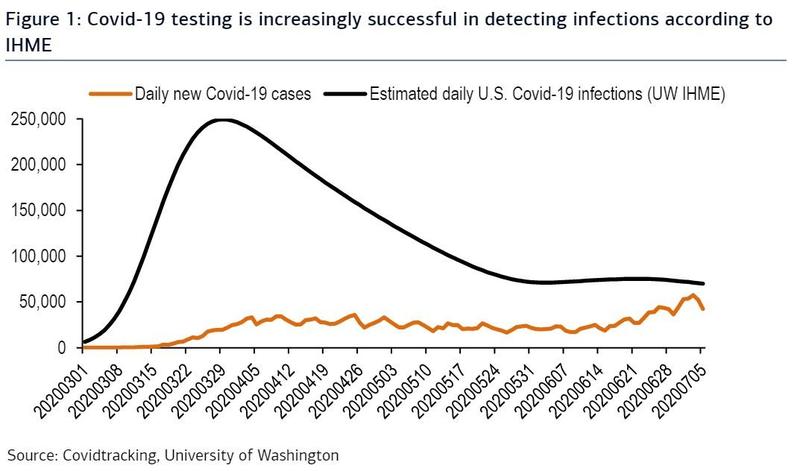
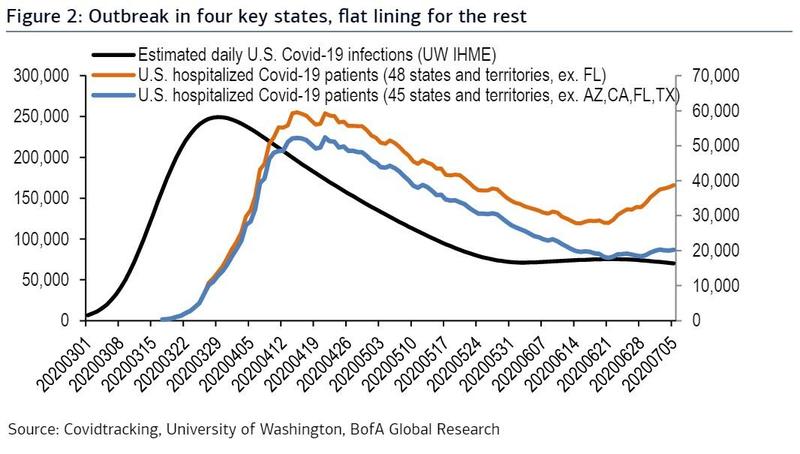
A similar proportion of patients responded to behavioral therapy and zolpidem (Ambien) as first-line therapy for insomnia disorder, but the proportion of responders diverged with second-line treatments, a randomized trial found.
Among 211 adults with chronic insomnia, 45.5% of patients responded to 6 weeks of behavioral therapy as a first-line treatment, and response rates continued to improve among patients who were then assigned to cognitive therapy (50.1% to 68.2%) or 5-10 mg zolpidem (40.6% to 62.7%) as a second-line treatment, reported Charles M. Morin, PhD, of the Université Laval in Quebec, Canada, and colleagues.
As noted in the study online in JAMA Psychiatry, participants were randomized to receive either behavioral therapy or zolpidem for 6 weeks, and remitters remained on maintenance therapy for the next 12 months. Meanwhile, non-remitters continued on to second-stage therapy, trazodone or cognitive therapy, for a total of four treatment arms.
“The proportion of patients who responded to 6 weeks of zolpidem as a first-line therapy was similar to those assigned to behavioral therapy (49.7%), but patients who were started on zolpidem did best when they continued with 50-150 mg trazodone (46.4% to 55.7%) rather than continuing to cognitive therapy,” the researchers reported.
However, no significant differences were observed in analyses comparing the cumulative response rates of all four treatment options, the team added.
“That still leaves about 25-30% of patients who, even with two treatments, did not respond,” Morin told MedPage Today. “I think we moved one step further from where we were before but we still don’t have the answers for all patients with chronic insomnia.”
The American College of Physicians recommends cognitive behavioral therapy as a first-line treatment for chronic insomnia, and pharmacologic therapy if that is ineffective. The American Academy of Sleep Medicine has also published recommendations on the most appropriate drugs to use in various settings, including zolpidem for sleep onset and sleep maintenance insomnia.
In the clinical setting, patients often try multiple treatments for insomnia and are commonly prescribed medication before coming in for behavioral or cognitive interventions, commented Michael A. Grandner, PhD, director of the Behavioral Sleep Medicine Clinic at the University of Arizona, who was not involved with the study.
“These results suggest that patients should get behavioral or cognitive therapy first, and that this is the most efficient way to get the most people the best help possible,” Grandner told MedPage Today in an email. “It was also important to note that — consistent with previous studies — non-medication therapy worked at least as well as medication as a first-line therapy, without all of the risks involved.”
Although the study lacked a control group, all the treatments have already demonstrated efficacy versus placebo, so comparing them with one another instead is “not a major problem,” in this context, Grandner commented.
It should also be noted that in real-world settings, many patients are on multiple treatments at once, instead of sequential treatments like in this study, he added. “This made for a cleaner analysis, but the real world is messier.”
The cohort — mean age of 46, 63% of whom were female — was enrolled through advertisements in clinics and the community at the Université Laval in Quebec and National Jewish Health in Denver. Participants were mostly white (86%), and 25% had tried a sleep-promoting medication within the past year.
Remission was defined as scores of less than 8 on the Insomnia Severity Index, while a treatment response was defined as a change of seven or more points, the researchers reported.
While behavioral therapy aims to change sleep habits, cognitive therapy focuses more on a patient’s beliefs and attitudes towards sleep, and is more time-intensive, Morin said. “That’s why we offered it only as a second-line treatment, if people did not respond to the more straightforward behavioral therapy.”
The proportion of patients who met remission criteria after first-line therapy was similar between the behavioral therapy and zolpidem arms (39% vs 30.3%), but the only second-line treatment pairing that was associated with significantly improved remission rates was behavioral therapy followed by zolpidem (39.1% to 55.9%), the investigators said.
Among 74 patients with preexisting psychiatric conditions (most commonly anxiety or depression), response rates were highest with two treatments of the same modality, such as behavioral therapy followed by cognitive therapy (58.3% to 85.3%) or zolpidem followed by trazodone (40.0% to 77.5%).
Notably, a higher proportion of patients in the pharmacologic arm dropped out of the trial compared with patients started on behavioral therapy (P=0.01), indicating that cognitive and behavioral therapies are more tolerable and may be preferred by some patients, Morin said.
Overall, a higher proportion of patients on zolpidem in the first treatment phase reported cognitive problems with attention, concentration, and memory compared with patients assigned to behavioral therapy (16% vs 7.4%), the researchers reported. However, a higher proportion of patients on behavioral therapy as first-line treatment experienced daytime sleepiness compared with those on zolpidem (13% vs 4%).
“The nuance here is that those who received a second treatment did best when that second treatment involved either cognitive therapy or trazodone,” Morin said. “We believe the reason why these patients did better is that cognitive therapy is not just focused on sleep — it also addresses mood disturbances — and trazodone is an antidepressant, so it is therefore more likely to improve mood.”
Disclosures
The study was funded by the National Institute of Mental Health.
Morin reported financial relationships with Abbot, Eisai, Merck, Philips, Weight Watchers, Idorsia, and Canopy Health; co-authors also reported many ties with industry.
Primary Source
JAMA Psychiatry