Lara Bull-Otterson, PhD1,2; Elizabeth B. Gray, MPH2; Daniel S. Budnitz, MD3,4; Heather M. Strosnider, PhD1,5; Lyna Z. Schieber, MD, DPhil1,6; Joseph Courtney, PhD1,5; Macarena C. García, DrPH1,7; John T. Brooks, MD8; William R. Mac Kenzie, MD1,7; Adi V. Gundlapalli, MD, PhD1,9 (View author affiliations)
Summary
What is already known about this topic?
Hydroxychloroquine and chloroquine are approved to treat autoimmune diseases and to prevent and treat malaria. Earlier this year, they were widely reported to be of potential benefit in the prevention and treatment of COVID-19; however, current data indicate that the potential benefits of these drugs do not outweigh their risks.
What is added by the report?
New prescriptions by specialists who did not typically prescribe these medications (defined as specialties accounting for ≤2% of new prescriptions before 2020) increased from 1,143 prescriptions in February 2020 to 75,569 in March 2020, an 80-fold increase from March 2019.
What are the implications for public health practice?
Attention to updated clinical guidance, especially by nonroutine prescribers, will help safeguard supplies and ensure safe use of hydroxychloroquine and chloroquine for patients with approved indications.
FIGURE 2. Estimated new retail prescriptions of hydroxychloroquine or chloroquine dispensed, by prescriber category* — United States, January–June, 2019–2020
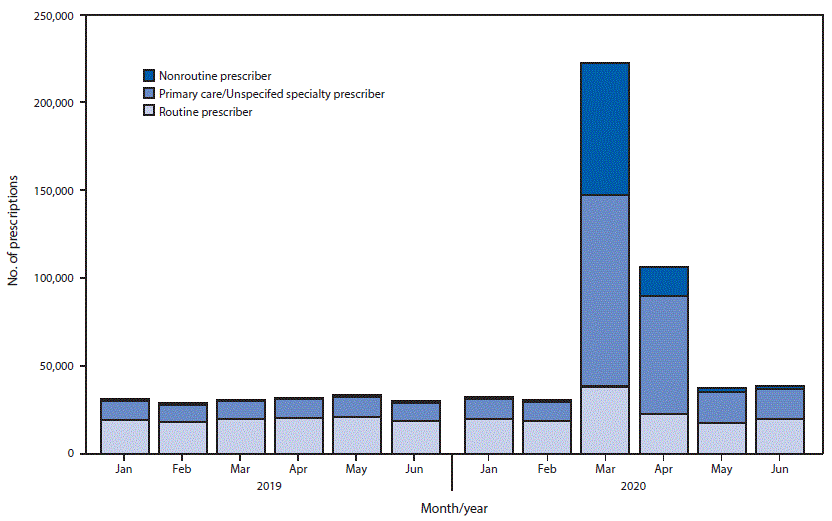
* Nonroutine prescribers = addiction medicine, allergy/immunology, anesthesiology, cardiology, cardiothoracic surgery, cardiovascular surgery, clinical neurophysiology, clinical pharmacology, colon and rectal surgery, critical care, critical care medicine, dentistry, dermatopathology, diagnostic laboratory, diagnostic laboratory immunology, emergency medicine, endocrinology, gastroenterology, general preventive medicine, general surgery, genetics, geriatric psychiatry, geriatrics, hematology, hepatology, hospice and palliative medicine, infectious disease, medical microbiology, naturopathic doctor, neurological surgery, neurology, neurosurgery-critical care, nuclear medicine, nutrition, obstetrics/gynecology, obstetrics/gynecology-critical care, occupational medicine, oncology, ophthalmology, optometry, orthopedic surgery, orthopedic surgery of spine, other, other surgery, otolaryngology, otology, pain medicine, pathology, pediatric critical care, pediatric neurosurgery, pharmacist, physical medicine and rehab, plastic surgery, podiatry, psychiatry, psychology, pulmonary critical care, pulmonary diseases, radiology, sleep medicine, sports medicine, surgery, thoracic surgery, and urology. Primary care/unspecified prescribers = family practice, general practice, internal medicine, internal medicine/pediatrics, nurse practitioner, osteopathic medicine, pediatrics, physician assistant, and specialty unspecified. Routine prescribers = allergy, dermatology, nephrology, and rheumatology.