Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I'm Dr F. Perry Wilson of the Yale School of Medicine.
There's a meme flying around the interwebs that COVID vaccines might render young people infertile. Taken on its own, this may seem like run-of-the-mill antivax fearmongering — and it is, but this one seems to have some legs. In fact, a UK survey found that one quarter of young women would decline the vaccine, citing concerns about fertility.
This is actually a sort of old vaccine trope. It's been trotted out — without any evidence — for the polio vaccine and the HPV vaccine. And I get why it's so powerful. Fertility is obviously a huge issue, a basic human function. But it also immediately conjures up the long term: Sure, I may be protected from COVID today, but what if I want to have kids 15 years from now and find out I can't? The Handmaid's Tale stuff. Disturbing.
So I want to show how this thing got started, but more importantly, I want to make an argument: that if you really want to worry about fertility, you should worry about SARS-CoV-2 more than the SARS-CoV-2 vaccine.
You can trace the earliest emergence of this idea to two guys: Wolfgang Wodarg, a physician and German politician; and Michael Yeadon, an ex-Pfizer scientist. Yeadon's Pfizer link lent him credibility, though a perusal of his Twitter account (now deleted) suggests that he was not the best COVID prognosticator. This was tweeted a couple months before the UK's much more deadly second wave.
In any case, their argument centered around the similarity between the spike protein that the vaccines code for and syncytin-1, a human protein critical for placental development. Syncytin-1 is known as an endogenous retroviral element — basically, code in our DNA that came from an old virus. We have a bunch of these, actually. This one probably got in there around 25 million years ago and conferred some selective advantage, leading it to hang around.
The question: Is syncytin-1 similar enough to the spike protein of coronavirus that cross-reactive antibodies might attack the placenta?
The answer, at least according to the geneticists and immunologists I polled, is not really.
They are dramatically different proteins.
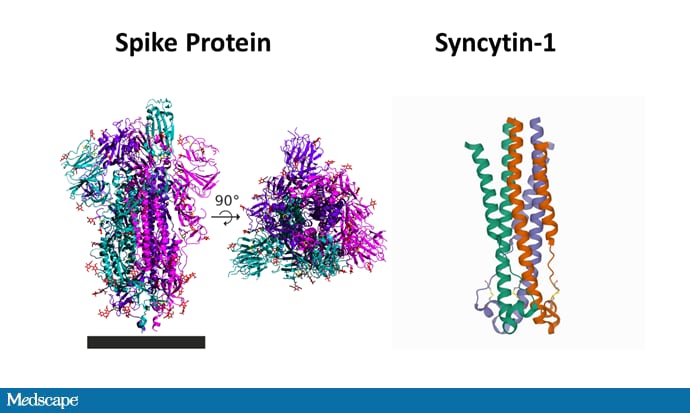
The spike protein is complex, with 1273 amino acids. Syncytin-1 has 538 amino acids. An alignment search suggests about a 7% overlap. Here's a sample of the sequences to give you a sense of what that means; syncytyin-1 is red, and the matches with spike protein are in red underneath.

But I'm told that this amount of homology is not surprising. More importantly, it's not really the degree of overlap that matters to figure out if an antibody will be cross-reactive. It's actually quite hard to predict and has to do with 3D topology of proteins and stuff.
If you want to figure out whether an antibody will be cross-reactive, just measure it. That's what Akiko Iwasaki here at Yale did. Her lab tested serum of women with COVID-19 and found no antibodies that bound to syncytin-1.
We also have the empirical data from the vaccine trials that showed no difference in miscarriage rates between women who became pregnant in the vaccine groups vs the placebo groups. We also now have data on over 100,000 pregnant women who have received the vaccine in the US. So far, no safety signals have emerged. This fertility theory just doesn't hold up to reality.
And not to be pedantic here, but even if there was homology between the spike protein and a human protein, the virus itself has the spike protein too, as well as a bunch of other proteins not present in the vaccine that might also have antibody cross-reactivity problems. Maybe it's better not to get COVID at all?
In fact, if it's sterility you're worried about, I honestly think there's more to be concerned about with the virus itself than the vaccine. Here's why.
The cellular receptor for the coronavirus is ACE2. Enter that into a protein expression atlas of your choice and look where ACE2 is most robustly expressed.
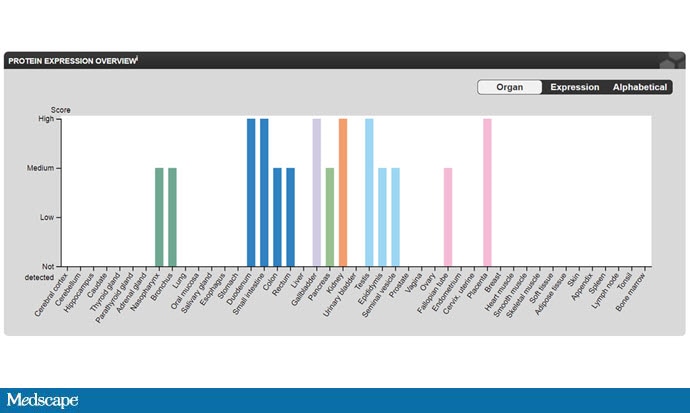
Source: The Human Protein Atlas
Nasopharynx and bronchus — no surprises there. But also, testis and placenta. In fact, ACE2 is robustly expressed on Sertoli cells, which are the support cells that help sperm be produced.
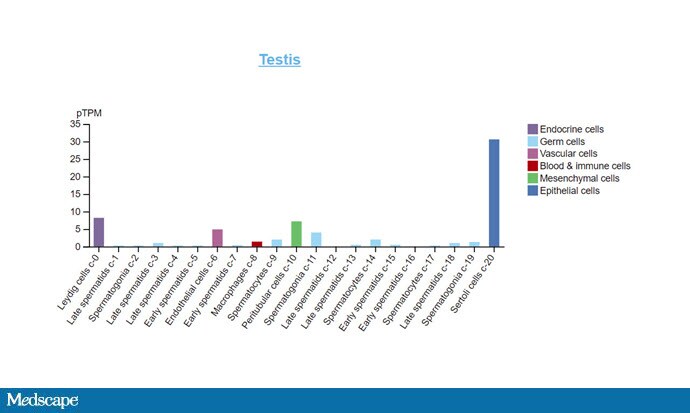
Source: The Human Protein Atlas
This has led to multiple papers (all hypothetical at this point) speculating that SARS-CoV-2 infection may impact male fertility.
To be fair, we haven't seen much evidence of this either. I have found a total of one paper — a case report — suggesting that COVID infection hurt a man's sperm count.
But the point is this: The vaccines prime your immune system against the spike protein. But so does COVID. And COVID, unlike the vaccine, can actually infect cells and kill them — including, potentially, cells that are important for reproduction.
The anti-vaccination crowd wants to argue that there is biologic plausibility for a COVID vaccine–infertility link. If that's our standard for evidence, there is a heck of a lot more plausibility for a COVID-infertility link. But as I've said many times before, biologic plausibility is just the start of research. Empirical data are necessary in the end. There's enough empirical data to conclude that it is highly unlikely that the vaccines will have any effect on fertility. It's pretty unlikely that COVID itself will have an effect either, but it seems to me like that possibility deserves some deeper investigation.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale's Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com.
https://www.medscape.com/viewarticle/952641