Search This Blog
Wednesday, May 14, 2025
Sanofi to invest at least $20 billion in the US through 2030
Sanofi today announces its intention to invest at least $20 billion in the United States through 2030. The expected investment includes a significant increase in research and development spending and the allocation of billions of dollars to US manufacturing.
The announcement comes as Sanofi prepares for the potential launch of numerous new first- or best-in-class medicines across many indications in the coming years, aiming to improve the health of countless Americans. Work being done within the United States by Sanofi and by the hundreds of companies it supports is anticipated to contribute to job creation and innovation in numerous communities that are home to Sanofi and its partners, while also helping to enhance the US supply chain.
Paul Hudson
Chief Executive Officer
"Sanofi's 13,000 US-based employees are pioneering the research and development of first- and best-in-class medicines across numerous therapeutic areas. Our expected investments in the US will be substantial and will help ensure the production of key medicines in the US."
Of the total investment, Sanofi will substantially increase spending in the US on R&D to accelerate its science. The company also plans to expand its US manufacturing capacity, both through direct investments in Sanofi sites, as well as through partnerships with other domestic manufacturers, to help ensure the production of medicines in the US. While Sanofi's investment decisions will be adjusted as the external environment continues to evolve, the planned investments are expected to create a significant number of high-paying jobs in multiple states in the coming years.
Boeing Scores Largest-Ever Order As Dealmaking Trump Tours Gulf States
President Trump's Gulf tour began Tuesday with the lifting of long-standing U.S. sanctions on Syria and a massive $600 billion investment deal from Saudi Arabia to invest in the American economy. The visit quickly led to a flurry of other investments, including multibillion-dollar AI deals between U.S. chipmakers Nvidia and AMD with Humain, a newly launched artificial intelligence firm backed by Saudi Arabia's Public Investment Fund. On Wednesday, the momentum continued in Qatar, where Trump unveiled Boeing's largest widebody aircraft order ever.
Faisal Al-Mudahka, editor-in-chief of the Gulf Times, described Qatar Airways' massive purchase of between 160 - 200 widebody aircraft from Boeing—the largest order in the company's history—as a "win-win" for both nations.
"I think Donald Trump and Qatar know how to package things to make political gains and economic gains," Al-Mudahka said.
Qatar Airways has become one of the world's top airlines, with a growing market share worldwide. The expanded fleet of the 787 Dreamliner model will allow the airline to add more long-haul flights.
Earlier, Trump participated in a signing ceremony on a series of bilateral agreements with Qatar's Emir Sheikh Tamim bin Hamad Al Thani in Doha, including:
Qatar Airways signed an agreement to purchase 210 787 Dreamliner and 777X aircraft powered by GE Aerospace engines.
A number of defence agreements, including a letter of intent on defence cooperation and a letter offer and acceptance for MQ-9B drones.
A joint declaration of cooperation between the two nations.
After the signing ceremony, Qatar's emir said he had a "great" few hours with Trump, during which they discussed various topics.
"I think after signing these documents, we are going to another level of relations," the emir said.
Trump also congratulated Boeing CEO Kelly Ortberg, who was also in Doha, for the signing of the deal.
Here's the White House's fact sheet of the agreement with Qatar to generate at least $1.2 trillion in economic activity between both countries:
The landmark deals celebrated today will drive innovation and prosperity for generations, bolster American manufacturing and technological leadership, and put America on the path to a new Golden Age.
Since President Trump took office, his commitment to American manufacturing and innovation has attracted trillions of dollars in investments and global commercial deals. Allies like Qatar are partnering in the United States' success.
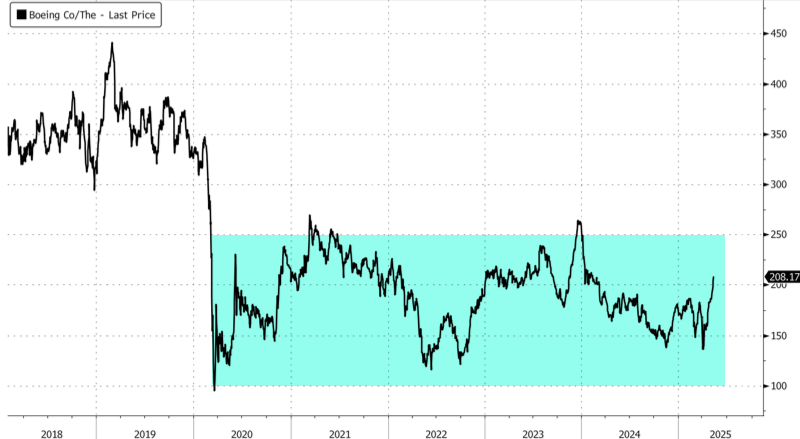
Boeing shares rose 2% in the late morning cash session in New York, trading around the $208 handle—the highest level in roughly 15 months
On Tuesday, at the start of Trump's trip, Saudi Arabia's sovereign wealth fund announced a $4.8 billion Boeing order.
President Trump is expected to return to the U.S. later this week, ready to boast about the mega-deals signed with Gulf states—deals he's likely to frame as part of the agenda to ascend America into a new Golden Age. This strategy comes as easing trade tensions between the U.S. and China has helped the stock market recover much of the recent trade war-related losses.
The media strategy from the White House appears clear: build and sustain momentum with positive economic news that squeezes left-leaning corporate media out of the conversation because of their persistent misinformation and disinformation campaigns.
Announcement On COVID-19 Vaccines For Kids Coming Soon: FDA Commissioner
by Zachary Stieber via The Epoch Times,
Health officials are poised to make an announcement on COVID-19 vaccines, the commissioner of the Food and Drug Administration (FDA) said.
“That is something that’s being discussed right now,” Dr. Marty Makary, the commissioner, said in an interview, released on May 12, after being asked by political activist Charlie Kirk about whether COVID-19 vaccines will remain on the childhood vaccination schedule.
“I think you’re going to see some announcement on that in the coming weeks, but I know they are trying to review all of the scientific data.”
The Centers for Disease Control and Prevention and the Department of Health and Human Services, which maintains the schedule, did not respond to a request for comment.
Makary told Kirk that there’s no evidence available at this time that supports giving healthy children additional COVID-19 vaccine doses.
“That evidence does not exist, and so we’re not going to rubberstamp things at the FDA,” he said.
“I don’t think you’re going to see the CDC pushing COVID shots in young, healthy children.”
The FDA commissioner, who expressed concern before joining the agency about vaccinating children, noted that there is no data from randomized, controlled trials for the COVID-19 vaccines that are currently available. The regulatory agency cleared the vaccines from Moderna, Pfizer, and Novavax in 2024, pointing to animal testing and data from previous versions of the shots.
“There’s no good randomized control data that the current version, the latest formulation, of the COVID shot, is necessary for young, healthy children. Other leading countries in Europe have recommended against it,” Makary said.
“So I think you’re going to hear something forthcoming.”
The CDC, in 2023, added the COVID-19 vaccines to the vaccination schedule, following a recommendation from its vaccine advisory panel.
The same panel said in April that it is leaning toward narrowing the current universal recommendation for COVID-19 vaccination. In the same meeting, officials noted that the United Kingdom and Australia are among the countries that do not recommend COVID-19 vaccine boosters for healthy children.
Health Secretary Robert F. Kennedy Jr. said that same month that officials were considering removing the COVID-19 vaccines from the schedule. Makary has said he would support that move.
The CDC, which has an acting director at present, has not commented on the prospect of the schedule being adjusted.
The American Academy of Pediatrics is among the organizations that support the current COVID-19 vaccine recommendations. The group says on its site that the vaccines are safe and effective.
The FDA is set to meet with its vaccine advisers on May 22 to discuss selecting updates to the COVID-19 vaccines, with Novavax saying it plans to participate in the meeting. Pfizer and Moderna have not responded to queries.
https://www.zerohedge.com/covid-19/announcement-covid-19-vaccines-kids-coming-soon-fda-commissioner
AbbVie Dives Further Into siRNA With $335M Upfront in ADARx Deal
The ADARx Pharmaceuticals partnership, which could be worth “several billion dollars” in the end, adds to AbbVie’s existing work in the space after the $1.4 billion acquisition of Aliada Therapeutics in October 2024.
AbbVie dipped its toes into the world of small interfering RNA last year with the buyout of Aliada Therapeutics. Now, the Illinois pharma is diving in further with a $335 million upfront payment to work with ADARx Pharmaceuticals to find new treatments.
The deal spans disease areas but neuroscience, immunology and oncology were noted in the Wednesday release as focus areas. ADARx will receive the upfront fee and also be eligible for “several billion dollars” in milestones down the line, the companies said.
ADARx will use its siRNA technology to find new molecules, with AbbVie contributing expertise in antibody engineering, antibody drug conjugates and tissue delivery. siRNA medicines regulate gene expression and protein production and can prevent the production of disease-causing proteins by targeting messenger RNA (mRNA) that encodes for these proteins.
The ADARx partnership adds to AbbVie’s existing work in the space after its $1.4 billion acquisition of Aliada Therapeutics in October 2024. That deal was focused on central nervous system disorders, particularly the anti-pyroglutamate amyloid beta (3pE-Aβ) antibody ALIA-1758 for Alzheimer’s disease.
Beyond the AbbVie partnership, ADARx has a pipeline of clinical-stage medicines for complement-mediated, cardiovascular and thrombotic diseases. The most advanced is ADX-324, which is nearing Phase III testing for hereditary angioedema. The biotech also has programs in discovery for obesity and neurodegeneration.
ADARx last raised funds in August 2023, taking home $200 million in series C funds to advance ADX-324 as well as ADX-038, which is in development for multiple complement-mediated diseases.
siRNA has become a hot space for pharma. Also on Wednesday, GSK inked a deal to buy Boston Pharmaceuticals’ efimosfermin alfa. The plan is to combine the FGF21 analog with GSK’s siRNA therapy GSK’990.
The new deals follow Eli Lilly’s March announcement that its siRNA therapy lepodisiran reduced lipoprotein(a) levels by nearly 94% in patients at risk of cardiovascular events such as heart attack or stroke. And Boehringer Ingelheim signed a deal in January 2024 worth up to $2 billion with Chinese biotech Suzhou Ribo Life Science and its Swedish unit Ribocure Pharmaceuticals to develop siRNA-based treatments for metabolic dysfunction-associated steatohepatitis.
https://www.biospace.com/deals/abbvie-dives-further-into-sirna-with-335m-upfront-in-adarx-deal
Call goes out for feedback on US health deregulation
President Donald Trump's push to deregulate the US federal governance system has stepped up a gear, and the administration wants the public to join in the effort to simplify health regulations.
"If you know a regulation that's making our health system worse, tell us," says a notice from the federal government, which has set up a page for comments to be submitted at its regulations.gov website for 60 days.
The drive revolves around a "10-for-one" policy laid out in a Trump executive order at the end of January, which demanded that for every new regulation proposed, at least 10 would be rescinded, with the net cost of all new rulemaking "less than zero."
It's not clear what the rationale or justification is for that ratio, although Trump has claimed he achieved a five-for-one cull in his first term in office. The new drive comes with an expanded scope that covers not only formal regulations but also "guidance documents, memoranda, policy statements, and similar directives."
The argument is that American companies and citizens are beset by unwarranted regulations that stifle competition and innovation, hamper economic growth, and drive up prices for consumers.
"This initiative is about restoring common sense to health care regulation," said FDA Commissioner Marty Makary in a statement.
"By cutting outdated red tape, we can lower costs, increase access to innovation, and let clinicians spend more time with patients – not paperwork," he added. "We welcome public input to help identify reforms that truly make a difference."
Health and Human Services (HHS) Secretary Robert F Kennedy Jr claimed in a video to introduce the request for information (RFI) that larger companies with greater resources may have been "responsible for writing a lot of these regulations in the first place" as their complexity helps them to "maintain market monopolies and to keep lower-cost and more agile, innovative players off the field."
The RFI asks commentators to identify the rules that need to be rescinded, how it operates, the reasons why it is not fit-for-purpose – for example because its costs outweigh its benefits, it no longer reflects the current state of technology, or is "bad policy, unreasoned, or unsound."
Ban on fluoride prescription drug products
Meanwhile, the FDA has said it will impose a ban on ingestible fluoride prescription drug products for children, saying they have never been approved for marketing and may have deleterious effects on the gut microbiome.
The restriction will not apply to toothpaste or mouthwashes that contain fluoride, according to the regulator, which said studies have suggested a link between fluoride and "thyroid disorders, weight gain, and possibly decreased IQ."
Several US states have stopped fluoridation of drinking water, and the FDA pointed out that fluoride is not added to drinking water in most of Europe or other countries of the world.
In April, Kennedy – who has claimed for some time that fluoride is linked to a variety of health conditions – said he had asked the Centers for Disease Control and Prevention (CDC) to stop recommending fluoridation of water and was assembling a taskforce to look into the risks.
"For the same reason that fluoride may kill bacteria on teeth, it may also kill intestinal bacteria important for a child's health," commented Makary.
"I am instructing our Center for Drug Evaluation and Research to evaluate the evidence regarding the risks of systemic fluoride exposure from FDA-regulated paediatric ingestible fluoride prescription drug products to better inform parents and the medical community on this emerging area," he added.
"When it comes to children, we should err on the side of safety."
https://pharmaphorum.com/news/call-goes-out-feedback-us-health-deregulation
GSK pays $1.2bn for liver drug as it culls TIGIT programme
GSK decided to abandon a TIGIT-targeted drug for cancer this week, then immediately hatched a plan to replace it with a late-stage candidate for metabolic dysfunction-associated steatohepatitis (MASH).
The demise of iTeos Therapeutics-partnered TIGIT inhibitor belrestotug does not come as a complete surprise, given the well-documented challenges faced by companies developing this class of drug, although it does follow some promising results reported at last year's ESMO congress.
The company said it is canning belrestotug, and its $2 billion iTeos alliance, based on new data from phase 2 trials, "which did not meet the established efficacy criteria for continued development."
Enter efimosfermin alfa, described as a phase 3-ready, once-monthly injectable fibroblast growth factor 21 (FGF21) analogue with potential across steatotic liver diseases (SLD), including MASH and alcohol-related liver diseases (ALD).
The drug (formerly called BOS-580) is designed to prevent the characteristic fibrosis (scarring) seen with SLDs, which affect around 5% of the global population and have limited treatment options.
MASH is a form of non-alcoholic fatty liver disease (NAFLD) that affects between 6 million and 8 million people in the US alone and, for some time, has been billed as pharma's next big growth area. It is largely associated with obesity and an unhealthy diet and lifestyle, and is on the rise in industrialised nations, leading to estimates that it could present a market opportunity worth tens of billions of dollars a year.
GSK has agreed to pay $1.2 billion upfront to acquire rights to efimosfermin from Cambridge, Massachusetts-based Boston Pharmaceuticals, pledging another $800 million in milestone payments if the programme meets future objectives.
Efimosfermin was originally developed by Novartis, which stands to receive royalties on sales if it reaches the market.
GSK has said it is modelling a potential launch in 2029, and views the drug as a companion to GSK532990, a small, interfering RNA (siRNA) targeting HSD17B13, which is in phase 2 for both MASH (formerly known as NASH) and ALD.
Boston Pharma recently reported phase 2 results with efimosfermin in patients with moderate-to-advanced MASH, which showed the drug was able to reverse liver fibrosis and stop disease progression, while also improving other biomarkers like triglyceride and blood glucose levels.
GSK said that the drug could be used alongside other drugs being developed for MASH, including GLP-1 agonists. Novo Nordisk's GLP-1 drug semaglutide has also been shown to improve liver fibrosis in a phase 3 trial, setting up potential regulatory filings this year.
Currently, the only FDA-approved therapy for MASH is Madrigal Pharma's Rezdiffra (resmetirom), which got a green light last year.
"The FGF21 class has shown some of the most exciting data in MASH, including first-in-disease evidence of cirrhosis reversal, and efimosfermin has the potential to define a new standard-of-care with its monthly dosing and tolerability profile," said GSK's chief scientific officer, Tony Wood.
https://pharmaphorum.com/news/gsk-pays-12bn-liver-drug-it-culls-tigit-programme
EC's von der Leyen loses bid to stop disclosure of Pfizer texts
European Commission President Ursula von der Leyen has lost a lawsuit attempting to force her to disclose text messages with Pfizer chief executive Albert Bourla on COVID-19 vaccine procurement at the height of the pandemic.
Judges at the EU's General Court in Luxembourg have come down on the side of the New York Times and its journalist Matina Stevi in the dispute, which published an article in 2021 claiming that "personal diplomacy" had played a big part in the EU's order for 1.8 billion doses of Pfizer and BioNTech's Comirnaty COVID-19 vaccine.
The EU has come under criticism by activist groups, including Transparency International Global Health, which slammed what it says was secrecy surrounding the procurement of COVID-19 vaccines.
The judges ruled the Commission had no legal standing for its refusal to disclose the messages, although that verdict could go to appeal at the European Court of Justice (ECJ).
In 2022, the Commission said that "the search undertaken by the President's cabinet for relevant text messages corresponding to the request for access to documents has not yielded any results," which earned it a rebuke from the EU Ombudsman for "maladministration" and a warning that all EU institutions must ensure "accountability in an era of instant messaging."
It had earlier said that it did not consider that text messages fell under its internal criteria for document recording – a position that was rejected by the ombudsman.
Today, the judges said that the EC "has not sufficiently clarified whether the requested text messages were deleted and, if so, whether the deletion was done deliberately or automatically or whether the president's mobile phone had been replaced in the meantime."
In a statement, the court said: "By contrast, Ms Stevi and The New York Times have produced relevant and consistent evidence describing the existence of exchanges, in the form of text messages in particular, between the President of the Commission and the CEO of Pfizer in the context of the procurement of vaccines by the Commission from that company during the COVID-19 pandemic."
As a result, they have "succeeded in rebutting the presumption of non-existence and of non-possession of the requested documents," it added.
The Commission said it "will now closely study the General Court's decision and decide on next steps. To this effect, the Commission will adopt a new decision providing a more detailed explanation."
It continued: "Transparency has always been of paramount importance for the Commission and President von der Leyen. We will continue to strictly abide by the solid legal framework in place to enforce our obligations."
https://pharmaphorum.com/news/ecs-von-der-leyen-loses-bid-stop-disclosure-pfizer-texts