Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN) today announced positive topline results from a randomized, controlled Phase 3 clinical trial (BHV3000-303 or Study 303) evaluating the efficacy and safety of its Zydis® orally dissolving tablet (ODT) formulation of rimegepant, an oral calcitonin gene-related peptide (CGRP) receptor antagonist, for the acute treatment of migraine. In Study 303, rimegepant Zydis ODT statistically differentiated from placebo on the two co-primary endpoints as well as the first 21 consecutive primary and secondary outcome measures that were prespecified in hierarchical testing. Consistent with the two previous Phase 3 clinical trials, Study 303 met its co-primary registrational endpoints of pain freedom and freedom from most bothersome symptom (MBS) at 2 hours using a single dose (Table 1). Importantly, patients treated with the rimegepant Zydis ODT formulation began to numerically separate from placebo on pain relief as early as 15 minutes, and this difference was statistically significant at 60 minutes (p < 0.0001) (Figure 1). Additionally, a significantly greater percentage of patients treated with rimegepant Zydis ODT returned to normal functioning by 60 minutes as compared to placebo (p = 0.0025). Lasting clinical benefit was observed through 48 hours after a single dose of rimegepant on freedom from pain (p < 0.0001), pain relief (p < 0.0001), freedom from the most bothersome symptom (p = 0.0018), and freedom from functional disability (p < 0.0001). Superiority over placebo was also demonstrated in multiple other secondary endpoints. The vast majority of patients treated with rimegepant Zydis ODT (85%) did not use any rescue medications.
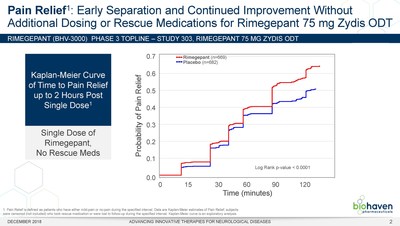
Table 1: Met Co-Primary Endpoints of Pain Freedom & Freedom from Most Bothersome Symptom
2 Hour Endpoint
|
Rimegepant(N=669)
|
Placebo(N=682)
|
Difference
|
p-value
|
Pain Freedom
|
21.2%
|
10.9%
|
10.3%
|
< 0.0001
|
Freedom from MBS1
|
35.1%
|
26.8%
|
8.3%
|
0.0009
|
1. Most Bothersome Symptom of Photophobia, Phonophobia or Nausea
| ||||
The safety and tolerability observations of rimegepant in Study 303 were consistent with the profile previously observed in Studies 301 and 302. Table 2 shows the pooled safety data across all three trials. In study 303, no single adverse event (AE) occurred in the rimegepant group with an incidence higher than 1.6% and overall rates of AEs were similar to placebo. With regard to liver function tests, one patient treated with placebo and one patient treated with rimegepant showed LFTs > 3x ULN in Study 303. Pooled liver function test results across the three pivotal trials (n=3,556) performed to date showed that rimegepant was similar to placebo with regard to aminotransferase (ALT or AST) levels above the upper limit of normal (ULN) and no patients experienced elevations in bilirubin > 2x ULN
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.