MyoKardia Inc MYOK 1.87%, a thinly traded mid-cap biotech, is on the radar of investors ahead of the company’s scheduled presentation at the European Society of Cardiology Congress Aug. 31-Sept. 4 in Paris.
The South San Francisco, California-based biotech, founded in 2012, uses a precision medicine approach to discover and develop targeted therapies for serious and neglected rare cardiovascular diseases.
Precision medicine integrates clinical and molecular information based on the biological basis of a disease in a bid to develop therapies.
Clinical Pipeline And Progress
MyoKardia’s pipeline boasts two candidates in the clinics and a few more in preclinical development.
The company is scheduled to present 36-week data from the PIONEER-OLE study — an open label extension study of the Phase 2 PIONEER study — that is evaluating its investigational drug and lead product candidate mavacamten for the treatment of symptomatic obstructive hypertrophic cardiomyopathy, or oHCM.
Mavacamten has a peak sales potential of $1.5 billion, according to Bank of America Merrill Lynch analyst Tazeen Ahmad.
HCM results from the enlargement of heart muscle cells, causing the thickening of the walls of the ventricles, specifically the left ventricle.
This thickening prevents the ventricle from expanding, and in turn reduces its pumping capacity and blocks blood flow out of the ventricle, causing oHCM.
Patients with oHCM are at an increased risk of heart failure, atrial fibrillation, stroke and death, including sudden cardiac death. About 410,000 people in the U.S. suffer from oHCM, out of which about 65,000 cases are diagnosed and are receiving medical attention, the company said.
In the Phase 2 PIONEER study, mavacamten achieved statistically significant changes in the primary endpoint of post-exercise left ventricular outflow tract, or LVOT, gradient from baseline. The study also achieved the key secondary endpoints of showing improvements in New York Heart Association, or NHYA, functional classification, exercise capacity as measured by peak oxygen consumption and change in dyspnea scores.
The PIONEER-OLE study enrolled 13 patients who previously completed the Phase 2 PIONEER-HCM trial.
MyoKardia announced positive 12- and 24-week safety, efficacy and biomarker data from the PIONEER-HCM trial in early March. Mavacamten is now being studied in a Phase 3 pivotal trial, EXPLORER-HCM, that has enrolled 220 patients randomized 1-to-1 to receive mavacamten or placebo for 30 weeks with a primary endpoint of clinical response.
MyoKardia is evaluating mavacameten for a second potential indication of symptomatic non-obstructive hypertrophic myocardiopathy, or nHCM in a Phase 2 study dubbed MAVERICK-HCM.
The company is also conducting a long-term extension study of patients completing Phase 2 MAVERICK-HCM or Phase 3 EXPLORER-HCM trials, which is named MAVA-LTE.
MyoKardia’s second pipeline asset, MYK-491, has completed two single-ascending dose Phase 1 studies in healthy volunteers and in patients with stable heart failure. It is being evaluated in patients with systolic heart failure, in which the left ventricle is too distended and weak to adequately pump blood.
The studies have shown that the investigational compound increased cardiac contracility by 5-20% across multiple echocardiographic parameters at high dose concentrations, with minimal impact on diastolic function.
MYK-491 is being evaluated in a Phase 2a multiple-ascending dose trial in patients with stable heart failure.
In addition, the company has several preclinical assets for the precision treatment of systolic and diastolic diseases. 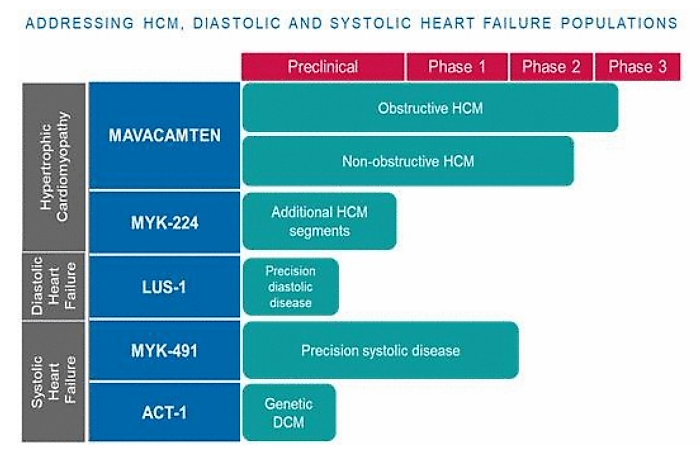

Source: MyoKardia
Going Solo
MyoKardia had a collaboration agreement with French pharma company Sanofi SA SNY 0.16% through the latter’s subsidiary Aventis. The deal was struck in 2014.
Through the collaboration, MyoKardia received about $230 million in funding from Sanofi over the years.
The companies decided not to extend the collaboration beyond the initial research term, which ended in December, and severed ties completely in April.
The decision helped MyoKardia regain the global rights to all its programs.
MyoKardia stock slid about 14% on the day the decision to go solo was announced, despite the company reassuring investors that it had nothing to do with clinical data or safety concerns.
The company said the move was precipitated by Sanofi’s desire to acquire the U.S. commercial rights to mavacamten, which it was unwilling to do.
“This was an important strategic step for us as we look ahead to mavacamten’s potential registration and commercial launch in the U.S. and the imminent advancement of MYK-224 into clinical studies,” the company said in a June press release.
Key Upcoming Catalysts
The pesentation of 36-week data from the PIONEER-OLE study at the European Society of Cardiology Congress is set for Aug. 31.
Data from the Phase 2 MAVERICK-HCM study in nHCM is due in the fourth quarter of 2019 .
EXPLORER-HCM topline data is slated for release in the second quarter of 2020, with the completion of enrollment expected in the second half of this year.
Competition
HCM has no FDA-approved therapy.
Symptomatic HCM is now treated with beta-blocker blood pressure medications, such as Propranolol, although this class of drug is not that effective and has off-target adverse effects as well. Therefore, patients more often use drugs indicated for hypertension, heart failure and other cardiovascular disorders.
Financials
The Sanofi partnership fetched MyoKardia revenue until 2018. The company has stepped up its spending on research and development expenses, as seen in the growth in the metric, which accelerated from about 33% in 2017 to about 53% in 2018. 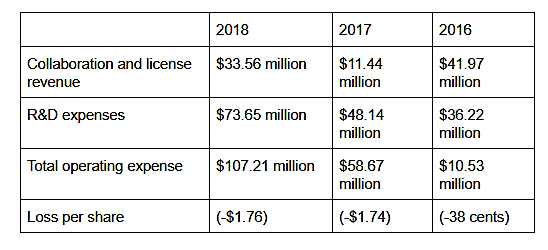

Source: MyoKardia 10-K filing
With the termination of the Sanofi deal, MyoKardia ceased reporting revenue.
For the six months ended June 2019, the company reported no revenue. On the other hand, its R&D expenses ballooned 60% year-over-year to $53.9 million.
MyoKardia will no longer receive R&D funding from Sanofi, which earlier had met 50% of mavacamten development costs and 100% of MYK-491 development costs.
MyoKardia reported a wider loss of $1.75 per share for the six months ended June 30 compared to a loss of 99 cents per share in the year-ago period.
As of June 30, the company had cash and cash equivalents as well as short-term investments totaling $558.49 million versus $314.69 million on Dec. 31, 2018.
The increase primarily reflects net proceeds of $271.2 million from a common stock offering in March.
Including long-term investments, the company has $602.4 million, which it said will be sufficient to meet the anticipated operating and capital expenditure requirements for the next 12 months.
Stock Take
MyoKardia shares, which were moving largely sideways in a broad range from their debut on Oct. 29, 2015 through early August 2017, received a shot in the arm following the release of topline results from the Phase 2 PIONEER-HCM study on Aug. 7, 2017.
From $17.15, the stock catapulted by about 83% to $31.45 in a single session. Subsequently, the stock was on a broader uptrend until late 2018 and saw some weakness thererafter.
The weakness was exacerbated following the termination of the Sanofi deal in early 2019. The stock found its footing following the release of fourth-quarter results in late February, but has been moving in a directionless manner since then. 

Source: Y Charts
A bullish crossover occurred in early August, with the shorter-term 50-day simple moving average or SMA, moving above the 200-day moving average.
The 14-day RSI, currently at 47.35, suggests the stock is in neutral territory.
The stock has downside support around its 50-day SMA, currently at $51.63, and its 200-day SMA, currently at $49.91.
If these levels are violated to the downside, the near-term support lies around the $45-$46 area.
The average analyst recommendation for MyoKardia shares is a Buy, and the average price target at $80.20, suggesting over 50% upside potential from current levels.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.